Critical role for IL-17A/F in the immunopathogenesis of obliterative airway disease induced by Anti-MHC I antibodies
- PMID: 23325004
- PMCID: PMC3549536
- DOI: 10.1097/TP.0b013e3182772244
Critical role for IL-17A/F in the immunopathogenesis of obliterative airway disease induced by Anti-MHC I antibodies
Abstract
Background: The IL-17 axis is implicated in pathogenesis of chronic rejection after human lung transplantation. Using a murine model of obliterative airway disease (OAD), we recently demonstrated that Abs to MHC class I antigens can induce immune responses to self-antigens that contributes to immunopathogenesis of chronic rejection. Using a murine model of OAD, we determined the role of IL-17 family members in induction of autoimmunity leading to OAD after ligation of MHC class I.
Methods: Anti-MHC class I or control antibodies (Abs) were administered intrabronchially to wild-type (WT) and IL-17a knock out (IL-17A-/-) C57BL/6.
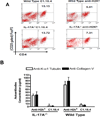
Results: By day 30, anti-MHC I administered endobronchially in IL-17A-/- mice demonstrated significant reduction in cellular infiltration, a 36.8% reduction in CD4 T cells, 62.7% in CD11b macrophages, 37.5% in degree of fibrosis, 1.94 fold and 2.17 fold decrease in anti-KAT and anti-Col-V, respectively, when compared with wild-type mice. Analysis of lung infiltrating cells in anti-MHC I WT revealed increase in IL-17A (KAT:92+21,Col-V:103+19spm) and IL-17F (KAT:5.03%,Col-V:2.75%) secreting CD4+ T cells. However, administration of anti-MHC I in IL-17A-/- demonstrated increase only in IL-17F for KAT (13.70%) and Col-V (7.08%). Anti-IL-17(A-F) mAb administration after anti-MHC I abrogated OAD in both WT and IL-17A-/-.
Conclusion: Our findings indicate that IL-17A and IL-17F secreted by CD4+Th17 cells specific to lung self-antigens are critical mediators of autoimmunity leading to the pathogenesis of OAD.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures







References
-
- Hachem RR, Khalifah AP, Chakinala MM, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80(10):1406. - PubMed
-
- Jaramillo A, Smith MA, Phelan D, et al. Temporal relationship between the development of anti-HLA antibodies and the development of bronchiolitis obliterans syndrome after lung transplantation. Transplant Proc. 1999;31(1–2):185. - PubMed
-
- Jaramillo A, Smith MA, Phelan D, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67(8):1155. - PubMed
-
- Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30(6):624. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

