Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease
- PMID: 23325084
- PMCID: PMC3633725
- DOI: 10.1038/ki.2012.439
Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease
Abstract
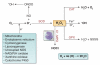
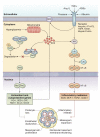
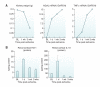
Oxidative stress and inflammation are mediators in the development and progression of chronic kidney disease (CKD) and its complications, and they are inseparably linked as each begets and amplifies the other. CKD-associated oxidative stress is due to increased production of reactive oxygen species (ROS) and diminished antioxidant capacity. The latter is largely caused by impaired activation of Nrf2, the transcription factor that regulates genes encoding antioxidant and detoxifying molecules. Protective effects of Nrf2 are evidenced by amelioration of oxidative stress, inflammation, and kidney disease in response to natural Nrf2 activators in animal models, while Nrf2 deletion amplifies these pathogenic pathways and leads to autoimmune nephritis. Given the role of impaired Nrf2 activity in CKD-induced oxidative stress and inflammation, interventions aimed at restoring Nrf2 may be effective in retarding CKD progression. Clinical trials of the potent Nrf2 activator bardoxolone methyl showed significant improvement in renal function in CKD patients with type 2 diabetes. However, due to unforeseen complications the BEACON trial, which was designed to investigate the effect of this drug on time to end-stage renal disease or cardiovascular death in patients with advanced CKD, was prematurely terminated. This article provides an overview of the role of impaired Nrf2 activity in the pathogenesis of CKD-associated oxidative stress and inflammation and the potential utility of targeting Nrf2 in the treatment of CKD.
Figures
References
-
- Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. 2003 Nov;12(6):593–8. - PubMed
-
- Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002 Nov;62(5):1524–38. - PubMed
-
- Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004 Jan;13(1):93–9. - PubMed
-
- Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol. 2004 Sep;24(5):469–73. - PubMed
-
- Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007 Nov;35(Pt 5):1147–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

