Temperature integration at the AC thermosensory neurons in Drosophila
- PMID: 23325228
- PMCID: PMC4135524
- DOI: 10.1523/JNEUROSCI.1894-12.2013
Temperature integration at the AC thermosensory neurons in Drosophila
Abstract
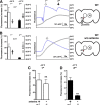
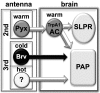
Temperature sensation has a strong impact on animal behavior and is necessary for animals to avoid exposure to harmful temperatures. It is now well known that thermoTRP (transient receptor potential) channels in thermosensory neurons detect a variable range of temperature stimuli. However, little is known about how a range of temperature information is relayed and integrated in the neural circuits. Here, we show novel temperature integration between two warm inputs via Drosophila TRPA channels, TRPA1 and Pyrexia (Pyx). The internal AC (anterior cell) thermosensory neurons, which express TRPA1, detect warm temperatures and mediate temperature preference behavior. We found that the AC neurons were activated twice when subjected to increasing temperatures. The first response was at ∼25°C via TRPA1 channel, which is expressed in the AC neurons. The second response was at ∼27°C via the second antennal segments, indicating that the second antennal segments are involved in the detection of warm temperatures. Further analysis reveals that pyx-Gal4-expressing neurons have synapses on the AC neurons and that mutation of pyx eliminates the second response of the AC neurons. These data suggest that AC neurons integrate both their own TRPA1-dependent temperature responses and a Pyx-dependent temperature response from the second antennal segments. Our data reveal the first identification of temperature integration, which combines warm temperature information from peripheral to central neurons and provides the possibility that temperature integration is involved in the plasticity of behavioral outputs.
Figures





Similar articles
-
The Drosophila TRPA1 Channel and Neuronal Circuits Controlling Rhythmic Behaviours and Sleep in Response to Environmental Temperature.Int J Mol Sci. 2017 Oct 3;18(10):2028. doi: 10.3390/ijms18102028. Int J Mol Sci. 2017. PMID: 28972543 Free PMC article. Review.
-
Temperature sensation in Drosophila.Curr Opin Neurobiol. 2015 Oct;34:8-13. doi: 10.1016/j.conb.2015.01.002. Epub 2015 Jan 21. Curr Opin Neurobiol. 2015. PMID: 25616212 Free PMC article. Review.
-
Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel.Cell Rep. 2012 Jan 26;1(1):43-55. doi: 10.1016/j.celrep.2011.11.002. Cell Rep. 2012. PMID: 22347718 Free PMC article.
-
Drosophila TRPA1 functions in temperature control of circadian rhythm in pacemaker neurons.J Neurosci. 2013 Apr 17;33(16):6716-25. doi: 10.1523/JNEUROSCI.4237-12.2013. J Neurosci. 2013. PMID: 23595730 Free PMC article.
-
Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster.Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14668-73. doi: 10.1073/pnas.0805041105. Epub 2008 Sep 11. Proc Natl Acad Sci U S A. 2008. PMID: 18787131 Free PMC article.
Cited by
-
An Automated Method to Determine the Performance of Drosophila in Response to Temperature Changes in Space and Time.J Vis Exp. 2018 Oct 12;(140):58350. doi: 10.3791/58350. J Vis Exp. 2018. PMID: 30371661 Free PMC article.
-
FlyMAD: rapid thermogenetic control of neuronal activity in freely walking Drosophila.Nat Methods. 2014 Jul;11(7):756-62. doi: 10.1038/nmeth.2973. Epub 2014 May 25. Nat Methods. 2014. PMID: 24859752
-
The Drosophila TRPA1 Channel and Neuronal Circuits Controlling Rhythmic Behaviours and Sleep in Response to Environmental Temperature.Int J Mol Sci. 2017 Oct 3;18(10):2028. doi: 10.3390/ijms18102028. Int J Mol Sci. 2017. PMID: 28972543 Free PMC article. Review.
-
Induction of aversive learning through thermogenetic activation of Kenyon cell ensembles in Drosophila.Front Behav Neurosci. 2014 May 15;8:174. doi: 10.3389/fnbeh.2014.00174. eCollection 2014. Front Behav Neurosci. 2014. PMID: 24860455 Free PMC article.
-
The role of PDF neurons in setting the preferred temperature before dawn in Drosophila.Elife. 2017 May 2;6:e23206. doi: 10.7554/eLife.23206. Elife. 2017. PMID: 28463109 Free PMC article.
References
-
- Altner H, Loftus R. Ultrastructure and function of insect thermo-and hygroreceptors. Annu Rev Entomol. 1985;30:273–295.
-
- Altner H, Routil C, Loftus R. The structure of bimodal chemo-, thermo-, and hygroreceptive sensilla on the antenna of Locusta migratoria. Cell Tissue Res. 1981;215:289–308. - PubMed
-
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. - PubMed
-
- Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574–1583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
