Two novel DEG/ENaC channel subunits expressed in glia are needed for nose-touch sensitivity in Caenorhabditis elegans
- PMID: 23325233
- PMCID: PMC3711640
- DOI: 10.1523/JNEUROSCI.2749-12.2013
Two novel DEG/ENaC channel subunits expressed in glia are needed for nose-touch sensitivity in Caenorhabditis elegans
Abstract
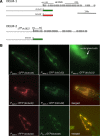
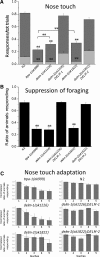
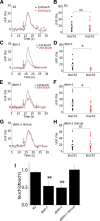
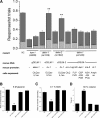
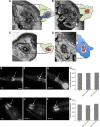
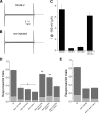
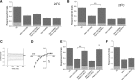
Neuronal DEG/ENaC (degenerin and epithelial Na(+) channel) Na(+) channels have been implicated in touch sensation. For example, MEC-4 is expressed in touch neurons in Caenorhabditis elegans and mediates gentle-touch response. Similarly, homologous mammalian ASIC2 and ASIC3 are expressed in sensory neurons and produce touch phenotypes when knocked out in mice. Here, we show that novel DEG/ENaC subunits DELM-1 and DELM-2 (degenerin-like channel mechanosensory linked-1 and degenerin-like channel mechanosensory linked-2) are expressed in glia associated with touch neurons in C. elegans and that their knock-out causes defects in mechanosensory behaviors related to nose touch and foraging, which are mediated by OLQ and IL1 sensory neurons. Cell-specific rescue supports that DELM-1 and DELM-2 are required cell-autonomously in glia to orchestrate mechanosensory behaviors. Electron microscopy reveals that in delm-1 knock-outs, OLQ and IL1 sensory neurons and associated glia are structurally normal. Furthermore, we show that knock-out of DELM-1 and DELM-2 does not disrupt the expression or cellular localization of TRPA-1, a TRP channel needed in OLQ and IL1 neurons for touch behaviors. Rather, rescue of the delm-1 nose-touch-insensitive phenotype by expression of a K(+) channel in socket glia and of a cationic channel in OLQ neurons suggests that DELM channels set basal neuronal excitability. Together, our data show that DELM-1 and DELM-2 are expressed in glia associated with touch neurons where they are not needed for neuronal structural integrity or cellular distribution of neuronal sensory channels, but rather for their function.
Figures


Similar articles
-
Mechanosensory molecules and circuits in C. elegans.Pflugers Arch. 2015 Jan;467(1):39-48. doi: 10.1007/s00424-014-1574-3. Epub 2014 Jul 23. Pflugers Arch. 2015. PMID: 25053538 Free PMC article. Review.
-
A glial DEG/ENaC channel functions with neuronal channel DEG-1 to mediate specific sensory functions in C. elegans.EMBO J. 2008 Sep 17;27(18):2388-99. doi: 10.1038/emboj.2008.161. Epub 2008 Aug 14. EMBO J. 2008. PMID: 18701922 Free PMC article.
-
The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel.Nature. 2002 Dec 12;420(6916):669-73. doi: 10.1038/nature01205. Nature. 2002. PMID: 12478294
-
Knockout of glial channel ACD-1 exacerbates sensory deficits in a C. elegans mutant by regulating calcium levels of sensory neurons.J Neurophysiol. 2012 Jan;107(1):148-58. doi: 10.1152/jn.00299.2011. Epub 2011 Oct 12. J Neurophysiol. 2012. PMID: 21994266 Free PMC article.
-
Touch sensitivity in Caenorhabditis elegans.Pflugers Arch. 2007 Aug;454(5):691-702. doi: 10.1007/s00424-006-0187-x. Epub 2007 Feb 7. Pflugers Arch. 2007. PMID: 17285303 Review.
Cited by
-
Structural Analysis of the Caenorhabditis elegans Dauer Larval Anterior Sensilla by Focused Ion Beam-Scanning Electron Microscopy.Front Neuroanat. 2021 Nov 5;15:732520. doi: 10.3389/fnana.2021.732520. eCollection 2021. Front Neuroanat. 2021. PMID: 34819841 Free PMC article.
-
Inferences of glia-mediated control in Caenorhabditis elegans.J Neurosci Res. 2021 May;99(5):1191-1206. doi: 10.1002/jnr.24803. Epub 2021 Feb 8. J Neurosci Res. 2021. PMID: 33559247 Free PMC article.
-
Pheromone sensing in Drosophila requires support cell-expressed Osiris 8.BMC Biol. 2022 Oct 11;20(1):230. doi: 10.1186/s12915-022-01425-w. BMC Biol. 2022. PMID: 36217142 Free PMC article.
-
[The effect of glial cells in the function and development of the nervous system in Caenorhabditis elegans].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016 May 25;45(3):315-22. doi: 10.3785/j.issn.1008-9292.2016.05.16. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 27651199 Free PMC article. Chinese.
-
Charging Up the Periphery: Glial Ionic Regulation in Sensory Perception.Front Cell Dev Biol. 2021 Aug 11;9:687732. doi: 10.3389/fcell.2021.687732. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458255 Free PMC article. Review.
References
-
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. - PubMed
-
- Altun ZF, Hall DH. Nervous system, neuronal support cells. WormAtlas. 2010
-
- Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. 2000;26:133–141. - PubMed
-
- Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
