A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis
- PMID: 23325234
- PMCID: PMC3566560
- DOI: 10.1523/JNEUROSCI.2949-12.2013
A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis
Abstract
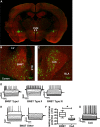
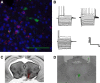
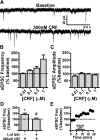
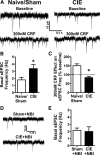
A growing literature suggests that catecholamines and corticotropin-releasing factor (CRF) interact in a serial manner to activate the bed nucleus of the stria terminalis (BNST) to drive stress- or cue-induced drug- and alcohol-seeking behaviors. Data suggest that these behaviors are driven in part by BNST projections to the ventral tegmental area (VTA). Together, these findings suggest the existence of a CRF-signaling pathway within the BNST that is engaged by catecholamines and regulates the activity of BNST neurons projecting to the VTA. Here we test three aspects of this model to determine: (1) whether catecholamines modify CRF neuron activity in the BNST; (2) whether CRF regulates excitatory drive onto VTA-projecting BNST neurons; and (3) whether this system is altered by ethanol exposure and withdrawal. A CRF neuron fluorescent reporter strategy was used to identify BNST CRF neurons for whole-cell patch-clamp analysis in acutely prepared slices. Using this approach, we found that both dopamine and isoproterenol significantly depolarized BNST CRF neurons. Furthermore, using a fluorescent microsphere-based identification strategy we found that CRF enhances the frequency of spontaneous EPSCs onto VTA-projecting BNST neurons in naive mice. This action of CRF was occluded during acute withdrawal from chronic intermittent ethanol exposure. These findings suggest that dopamine and isoproterenol may enhance CRF release from local BNST sources, leading to enhancement of excitatory neurotransmission on VTA-projecting neurons, and that this pathway is engaged by patterns of alcohol exposure and withdrawal known to drive excessive alcohol intake.
Figures


References
-
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. - PubMed
-
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. - PubMed
-
- Brown ZJ, Tribe E, D'souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. - PubMed
-
- Brown ZJ, Nobrega JN, Erb S. Central injections of noradrenaline induce reinstatement of cocaine seeking and increase c-fos mRNA expression in the extended amygdala. Behav Brain Res. 2011;217:472–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
