Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction
- PMID: 23325235
- PMCID: PMC3713715
- DOI: 10.1523/JNEUROSCI.2516-12.2013
Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction
Abstract
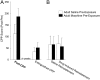
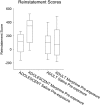
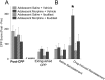
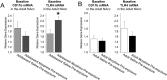
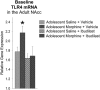
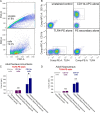
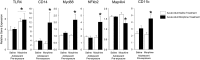
Adolescence in humans represents a unique developmental time point associated with increased risk-taking behavior and experimentation with drugs of abuse. We hypothesized that exposure to drugs of abuse during adolescence may increase the risk of addiction in adulthood. To test this, rats were treated with a subchronic regimen of morphine or saline in adolescence, and their preference for morphine was examined using conditioned place preference (CPP) and drug-induced reinstatement in adulthood. The initial preference for morphine did not differ between groups; however, rats treated with morphine during adolescence showed robust reinstatement of morphine CPP after drug re-exposure in adulthood. This effect was not seen in rats pretreated with a subchronic regimen of morphine as adults, suggesting that exposure to morphine specifically during adolescence increases the risk of relapse to drug-seeking behavior in adulthood. We have previously established a role for microglia, the immune cells of the brain, and immune molecules in the risk of drug-induced reinstatement of morphine CPP. Thus, we examined the role of microglia within the nucleus accumbens of these rats and determined that rats exposed to morphine during adolescence had a significant increase in Toll-like receptor 4 (TLR4) mRNA and protein expression specifically on microglia. Morphine binds to TLR4 directly, and this increase in TLR4 was associated with exaggerated morphine-induced TLR4 signaling and microglial activation in rats previously exposed to morphine during adolescence. These data suggest that long-term changes in microglial function, caused by adolescent morphine exposure, alter the risk of drug-induced reinstatement in adulthood.
Figures


Similar articles
-
Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression.J Neurosci. 2011 Dec 7;31(49):17835-47. doi: 10.1523/JNEUROSCI.3297-11.2011. J Neurosci. 2011. PMID: 22159099 Free PMC article.
-
Early adolescent nicotine exposure affects later-life cocaine reward in mice.Neuropharmacology. 2016 Jun;105:308-317. doi: 10.1016/j.neuropharm.2016.01.032. Epub 2016 Jan 22. Neuropharmacology. 2016. PMID: 26808314
-
Activation of p38 signaling in the microglia in the nucleus accumbens contributes to the acquisition and maintenance of morphine-induced conditioned place preference.Brain Behav Immun. 2012 Feb;26(2):318-25. doi: 10.1016/j.bbi.2011.09.017. Epub 2011 Oct 8. Brain Behav Immun. 2012. PMID: 22004988
-
Regulatory mechanisms and therapeutic potential of microglial inhibitors in neuropathic pain and morphine tolerance.J Zhejiang Univ Sci B. 2020 Mar.;21(3):204-217. doi: 10.1631/jzus.B1900425. J Zhejiang Univ Sci B. 2020. PMID: 32133798 Free PMC article. Review.
-
Targeting the Toll of Drug Abuse: The Translational Potential of Toll-Like Receptor 4.CNS Neurol Disord Drug Targets. 2015;14(6):692-9. doi: 10.2174/1871527314666150529132503. CNS Neurol Disord Drug Targets. 2015. PMID: 26022268 Free PMC article. Review.
Cited by
-
Neuroimmune Function and the Consequences of Alcohol Exposure.Alcohol Res. 2015;37(2):331-41, 344-51. Alcohol Res. 2015. PMID: 26695754 Free PMC article. Review.
-
Suicide, Psychoactive Substances, and Homelessness: A Scoping Review.Brain Sci. 2025 Jun 4;15(6):602. doi: 10.3390/brainsci15060602. Brain Sci. 2025. PMID: 40563776 Free PMC article. Review.
-
Chronic morphine-induced microRNA-124 promotes microglial immunosuppression by modulating P65 and TRAF6.J Immunol. 2015 Feb 1;194(3):1021-30. doi: 10.4049/jimmunol.1400106. Epub 2014 Dec 24. J Immunol. 2015. PMID: 25539811 Free PMC article.
-
Chronic exposure to ∆ 9-tetrahydrocannabinol in adolescence decreases social play behaviours.F1000Res. 2021 Nov 24;10:1191. doi: 10.12688/f1000research.53891.1. eCollection 2021. F1000Res. 2021. PMID: 34987774 Free PMC article.
-
Sex differences in neuroimmunity as an inherent risk factor.Neuropsychopharmacology. 2019 Jan;44(1):38-44. doi: 10.1038/s41386-018-0138-1. Epub 2018 Jun 29. Neuropsychopharmacology. 2019. PMID: 29977075 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
