Bone morphogenetic protein 4 mediates estrogen-regulated sensory axon plasticity in the adult female reproductive tract
- PMID: 23325243
- PMCID: PMC3581866
- DOI: 10.1523/JNEUROSCI.1704-12.2013
Bone morphogenetic protein 4 mediates estrogen-regulated sensory axon plasticity in the adult female reproductive tract
Abstract
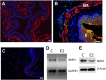
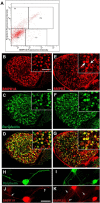
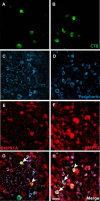
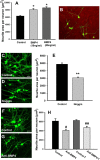
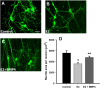
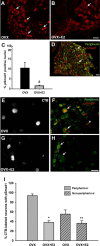
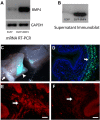
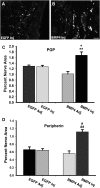
Peripheral axons are structurally plastic even in the adult, and altered axon density is implicated in many disorders and pain syndromes. However, mechanisms responsible for peripheral axon remodeling are poorly understood. Physiological plasticity is characteristic of the female reproductive tract: vaginal sensory innervation density is low under high estrogen conditions, such as term pregnancy, whereas density is high in low-estrogen conditions, such as menopause. We exploited this system in rats to identify factors responsible for adult peripheral neuroplasticity. Calcitonin gene-related peptide-immunoreactive sensory innervation is distributed primarily within the vaginal submucosa. Submucosal smooth muscle cells express bone morphogenetic protein 4 (BMP4). With low estrogen, BMP4 expression was elevated, indicating negative regulation by this hormone. Vaginal smooth muscle cells induced robust neurite outgrowth by cocultured dorsal root ganglion neurons, which was prevented by neutralizing BMP4 with noggin or anti-BMP4. Estrogen also prevented axon outgrowth, and this was reversed by exogenous BMP4. Nuclear accumulation of phosphorylated Smad1, a primary transcription factor for BMP4 signaling, was high in vagina-projecting sensory neurons after ovariectomy and reduced by estrogen. BMP4 regulation of innervation was confirmed in vivo using lentiviral transduction to overexpress BMP4 in an estrogen-independent manner. Submucosal regions with high virally induced BMP4 expression had high innervation density despite elevated estrogen. These findings show that BMP4, an important factor in early nervous system development and regeneration after injury, is a critical mediator of adult physiological plasticity as well. Altered BMP4 expression may therefore contribute to sensory hyperinnervation, a hallmark of several pain disorders, including vulvodynia.
Figures
References
-
- Abir R, Ben-Haroush A, Melamed N, Felz C, Krissi H, Fisch B. Expression of bone morphogenetic proteins 4 and 7 and their receptors IA, IB, and II in human ovaries from fetuses and adults. Fertil Steril. 2008;89:1430–1440. - PubMed
-
- Ai X, Cappuzzello J, Hall AK. Activin and bone morphogenetic proteins induce calcitonin gene-related peptide in embryonic sensory neurons in vitro. Mol Cell Neurosci. 1999;14:506–518. - PubMed
-
- Baleato RM, Aitken RJ, Roman SD. Vitamin A regulation of BMP4 expression in the male germ line. Dev Biol. 2005;286:78–90. - PubMed
-
- Berkley KJ, Robbins A, Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J Neurophysiol. 1993;69:533–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
