Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain
- PMID: 23325245
- PMCID: PMC6704888
- DOI: 10.1523/JNEUROSCI.3396-12.2013
Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain
Abstract
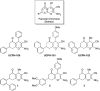
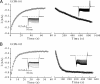
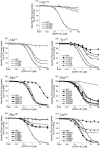
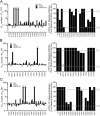
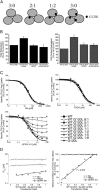
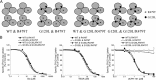
In the present study, the mechanism of action and molecular basis for the activity of the first class of selective inhibitors of the human excitatory amino acid transporter subtype 1 (EAAT1) and its rodent ortholog GLAST are elucidated. The previously reported specificity of UCPH-101 and UCPH-102 for EAAT1 over EAAT2 and EAAT3 is demonstrated to extend to the EAAT4 and EAAT5 subtypes as well. Interestingly, brief exposure to UCPH-101 induces a long-lasting inactive state of EAAT1, whereas the inhibition exerted by closely related analogs is substantially more reversible in nature. In agreement with this, the kinetic properties of UCPH-101 unblocking of the transporter are considerably slower than those of UCPH-102. UCPH-101 exhibits noncompetitive inhibition of EAAT1, and its binding site in GLAST has been delineated in an elaborate mutagenesis study. Substitutions of several residues in TM3, TM4c, and TM7a of GLAST have detrimental effects on the inhibitory potency and/or efficacy of UCPH-101 while not affecting the pharmacological properties of (S)-glutamate or the competitive EAAT inhibitor TBOA significantly. Hence, UCPH-101 is proposed to target a predominantly hydrophobic crevice in the "trimerization domain" of the GLAST monomer, and the inhibitor is demonstrated to inhibit the uptake through the monomer that it binds to exclusively and not to affect substrate translocation through the other monomers in the GLAST trimer. The allosteric mode of UCPH-101 inhibition underlines the functional importance of the trimerization domain of the EAAT and demonstrates the feasibility of modulating transporter function through ligand binding to regions distant from its "transport domain."
Figures

 ) or presence (●) of 10 μ
) or presence (●) of 10 μ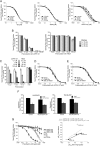





Similar articles
-
Caveolin-1 Sensitivity of Excitatory Amino Acid Transporters EAAT1, EAAT2, EAAT3, and EAAT4.J Membr Biol. 2016 Jun;249(3):239-49. doi: 10.1007/s00232-015-9863-0. Epub 2015 Dec 21. J Membr Biol. 2016. PMID: 26690923
-
Design, synthesis and pharmacological characterization of coumarin-based fluorescent analogs of excitatory amino acid transporter subtype 1 selective inhibitors, UCPH-101 and UCPH-102.Bioorg Med Chem. 2012 Dec 1;20(23):6831-9. doi: 10.1016/j.bmc.2012.09.049. Epub 2012 Oct 2. Bioorg Med Chem. 2012. PMID: 23072958
-
Bioavailability Studies and in vitro Profiling of the Selective Excitatory Amino Acid Transporter Subtype 1 (EAAT1) Inhibitor UCPH-102.ChemMedChem. 2016 Feb 17;11(4):403-19. doi: 10.1002/cmdc.201500527. Epub 2016 Jan 21. ChemMedChem. 2016. PMID: 26797816
-
GLAST But Not Least--Distribution, Function, Genetics and Epigenetics of L-Glutamate Transport in Brain--Focus on GLAST/EAAT1.Neurochem Res. 2015 Dec;40(12):2461-72. doi: 10.1007/s11064-015-1605-2. Epub 2015 May 14. Neurochem Res. 2015. PMID: 25972039 Review.
-
The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse?J Neurochem. 2006 Aug;98(4):1007-18. doi: 10.1111/j.1471-4159.2006.03978.x. Epub 2006 Jun 27. J Neurochem. 2006. PMID: 16800850 Review.
Cited by
-
Conserved allosteric inhibition mechanism in SLC1 transporters.Elife. 2023 Mar 1;12:e83464. doi: 10.7554/eLife.83464. Elife. 2023. PMID: 36856089 Free PMC article.
-
FN1 mediated activation of aspartate metabolism promotes the progression of triple-negative and luminal a breast cancer.Breast Cancer Res Treat. 2023 Oct;201(3):515-533. doi: 10.1007/s10549-023-07032-9. Epub 2023 Jul 17. Breast Cancer Res Treat. 2023. PMID: 37458908
-
Brain pericytes upregulate glutamate uptake by astrocytes in vitro through sodium-dependent transporter.IBRO Neurosci Rep. 2025 Jun 1;19:54-61. doi: 10.1016/j.ibneur.2025.05.017. eCollection 2025 Dec. IBRO Neurosci Rep. 2025. PMID: 40535312 Free PMC article.
-
Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease.Cell Mol Life Sci. 2015 Sep;72(18):3489-506. doi: 10.1007/s00018-015-1937-8. Epub 2015 Jun 2. Cell Mol Life Sci. 2015. PMID: 26033496 Free PMC article. Review.
-
Potential Mechanism of Cellular Uptake of the Excitotoxin Quinolinic Acid in Primary Human Neurons.Mol Neurobiol. 2021 Jan;58(1):34-54. doi: 10.1007/s12035-020-02046-6. Epub 2020 Sep 6. Mol Neurobiol. 2021. PMID: 32894500
References
-
- Bendahan A, Armon A, Madani N, Kavanaugh MP, Kanner BI. Arginine 447 plays a pivotal role in substrate interactions in a neuronal glutamate transporter. J Biol Chem. 2000;275:37436–37442. - PubMed
-
- Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. - PubMed
-
- Bunch L, Erichsen MN, Jensen AA. Excitatory amino acid transporters as potential drug targets. Expert Opin Ther Targets. 2009;13:719–731. - PubMed
-
- Conradt M, Stoffel W. Functional analysis of the high affinity, Na+-dependent glutamate transporter GLAST-1 by site-directed mutagenesis. J Biol Chem. 1995;270:25207–25212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
