Cortical control of affective networks
- PMID: 23325249
- PMCID: PMC3711588
- DOI: 10.1523/JNEUROSCI.0092-12.2013
Cortical control of affective networks
Abstract
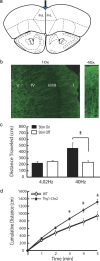
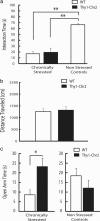
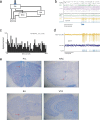
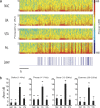
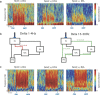
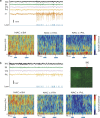
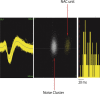
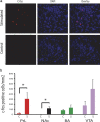
Transcranial magnetic stimulation and deep brain stimulation have emerged as therapeutic modalities for treatment refractory depression; however, little remains known regarding the circuitry that mediates the therapeutic effect of these approaches. Here we show that direct optogenetic stimulation of prefrontal cortex (PFC) descending projection neurons in mice engineered to express Chr2 in layer V pyramidal neurons (Thy1-Chr2 mice) models an antidepressant-like effect in mice subjected to a forced-swim test. Furthermore, we show that this PFC stimulation induces a long-lasting suppression of anxiety-like behavior (but not conditioned social avoidance) in socially stressed Thy1-Chr2 mice: an effect that is observed >10 d after the last stimulation. Finally, we use optogenetic stimulation and multicircuit recording techniques concurrently in Thy1-Chr2 mice to demonstrate that activation of cortical projection neurons entrains neural oscillatory activity and drives synchrony across limbic brain areas that regulate affect. Importantly, these neural oscillatory changes directly correlate with the temporally precise activation and suppression of limbic unit activity. Together, our findings show that the direct activation of cortical projection systems is sufficient to modulate activity across networks underlying affective regulation. They also suggest that optogenetic stimulation of cortical projection systems may serve as a viable therapeutic strategy for treating affective disorders.
Figures


References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
