Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance
- PMID: 23325250
- PMCID: PMC3711541
- DOI: 10.1523/JNEUROSCI.3082-12.2013
Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance
Abstract
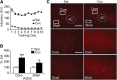
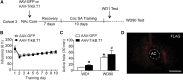
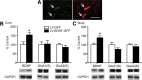
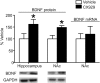
Brain-derived neurotrophic factor (BDNF) contributes to diverse types of plasticity, including cocaine addiction. We investigated the role of BDNF in the rat nucleus accumbens (NAc) in the incubation of cocaine craving over 3 months of withdrawal from extended access cocaine self-administration. First, we confirmed by immunoblotting that BDNF levels are elevated after this cocaine regimen on withdrawal day 45 (WD45) and showed that BDNF mRNA levels are not altered. Next, we explored the time course of elevated BDNF expression using immunohistochemistry. Elevation of BDNF in the NAc core was detected on WD45 and further increased on WD90, whereas elevation in shell was not detected until WD90. Surface expression of activated tropomyosin receptor kinase B (TrkB) was also enhanced on WD90. Next, we used viral vectors to attenuate BDNF-TrkB signaling. Virus injection into the NAc core enhanced cue-induced cocaine seeking on WD1 compared with controls, whereas no effect was observed on WD30 or WD90. Attenuating BDNF-TrkB signaling in shell did not affect cocaine seeking on WD1 or WD45 but significantly decreased cocaine seeking on WD90. These results suggest that basal levels of BDNF transmission in the NAc core exert a suppressive effect on cocaine seeking in early withdrawal (WD1), whereas the late elevation of BDNF protein in NAc shell contributes to incubation in late withdrawal (WD90). Finally, BDNF protein levels in the NAc were significantly increased after ampakine treatment, supporting the novel hypothesis that the gradual increase of BDNF levels in NAc accompanying incubation could be caused by increased AMPAR transmission during withdrawal.
Figures



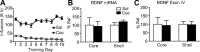



Similar articles
-
Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward.Biol Psychiatry. 2009 Apr 15;65(8):696-701. doi: 10.1016/j.biopsych.2008.09.032. Epub 2008 Nov 6. Biol Psychiatry. 2009. PMID: 18990365 Free PMC article.
-
Replication and extension of the subregion selectivity of glutamate-related changes within the nucleus accumbens associated with the incubation of cocaine-craving.Pharmacol Biochem Behav. 2024 Dec;245:173889. doi: 10.1016/j.pbb.2024.173889. Epub 2024 Oct 9. Pharmacol Biochem Behav. 2024. PMID: 39389205
-
Protein Translation in the Nucleus Accumbens Is Dysregulated during Cocaine Withdrawal and Required for Expression of Incubation of Cocaine Craving.J Neurosci. 2018 Mar 14;38(11):2683-2697. doi: 10.1523/JNEUROSCI.2412-17.2018. Epub 2018 Feb 5. J Neurosci. 2018. PMID: 29431650 Free PMC article.
-
Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving.Neuropharmacology. 2014 Jan;76 Pt B(0 0):287-300. doi: 10.1016/j.neuropharm.2013.04.061. Epub 2013 May 30. Neuropharmacology. 2014. PMID: 23727437 Free PMC article. Review.
-
Multiple faces of BDNF in cocaine addiction.Behav Brain Res. 2015 Feb 15;279:240-54. doi: 10.1016/j.bbr.2014.11.018. Epub 2014 Nov 15. Behav Brain Res. 2015. PMID: 25449839 Free PMC article. Review.
Cited by
-
Enhanced Cocaine-Associated Contextual Learning in Female H/Rouen Mice Selectively Bred for Depressive-Like Behaviors: Molecular and Neuronal Correlates.Int J Neuropsychopharmacol. 2015 Mar 2;18(8):pyv022. doi: 10.1093/ijnp/pyv022. Int J Neuropsychopharmacol. 2015. PMID: 25733538 Free PMC article.
-
Synaptic mechanisms underlying persistent cocaine craving.Nat Rev Neurosci. 2016 Jun;17(6):351-65. doi: 10.1038/nrn.2016.39. Epub 2016 May 6. Nat Rev Neurosci. 2016. PMID: 27150400 Free PMC article. Review.
-
Accumbens brain-derived neurotrophic factor (BDNF) transmission inhibits cocaine seeking.Addict Biol. 2019 Sep;24(5):860-873. doi: 10.1111/adb.12638. Epub 2018 Jun 11. Addict Biol. 2019. PMID: 29890020 Free PMC article.
-
The self-administration of rapidly delivered cocaine promotes increased motivation to take the drug: contributions of prior levels of operant responding and cocaine intake.Psychopharmacology (Berl). 2014 Oct;231(21):4241-52. doi: 10.1007/s00213-014-3576-4. Epub 2014 Apr 22. Psychopharmacology (Berl). 2014. PMID: 24752656
-
Cocaine-induced plasticity in the cerebellum of sensitised mice.Psychopharmacology (Berl). 2015 Dec;232(24):4455-67. doi: 10.1007/s00213-015-4072-1. Epub 2015 Oct 20. Psychopharmacology (Berl). 2015. PMID: 26482898
References
-
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–437. - PubMed
-
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. - PubMed
-
- Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned place preference and reinstatement in rats. Psychopharmacology. 2008;199:169–182. - PubMed
-
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
