Convergence of vestibular and neck proprioceptive sensory signals in the cerebellar interpositus
- PMID: 23325256
- PMCID: PMC3711745
- DOI: 10.1523/JNEUROSCI.3460-12.2013
Convergence of vestibular and neck proprioceptive sensory signals in the cerebellar interpositus
Abstract
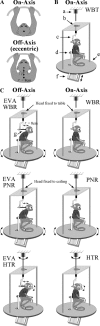
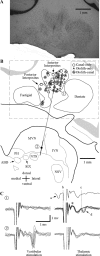
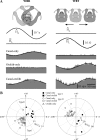
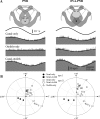
The cerebellar interpositus nucleus (IN) contributes to controlling voluntary limb movements. We hypothesized that the vestibular signals within the IN might be transformed into coordinates describing the body's movement, appropriate for controlling limb movement. We tested this hypothesis by recording from IN neurons in alert squirrel monkeys during vestibular and proprioceptive stimulation produced during (1) yaw head-on-trunk rotation about the C1-C2 axis while in an orthograde posture and (2) lateral side-to-side flexion about the C6-T3 axis while in a pronograde posture. Neurons (44/67) were sensitive to vestibular stimulation (23/44 to rotation and translation, 14/44 to rotation only, 7/44 to translation only). Most neurons responded during contralateral movement. Neurons (29/44) had proprioceptive responses; the majority (21/29) were activated during neck rotation and lateral flexion. In all 29 neurons with convergent vestibular and neck proprioceptive input those inputs functionally canceled each other during all combined sensory stimulation, whether in the orthograde or pronograde posture. These results suggest that two distinct populations of IN neurons exist, each of which has vestibular sensitivity. One population carries vestibular signals that describe the head's movement in space as is traditional for vestibular signals without proprioceptive signals. A second population of neurons demonstrated precise matching of vestibular and proprioceptive signals, even for complicated stimuli, which activated the semicircular canals and otolith organs and involved both rotation and flexion in the spine. Such neurons code body (not head) motion in space, which may be the appropriate platform for controlling limb movements.
Figures



References
-
- Anastasopoulos D, Mergner T, Becker W, Deecke L. Sensitivity of external cuneate neurons to neck rotation in three-dimensional space. Exp Brain Res. 1991;85:565–576. - PubMed
-
- Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol. 2000;84:2113–2132. - PubMed
-
- Arshavskiı̆ Iu I, Orlovskiı̆ GN, Pavlova GA. [Vestibular reactions of neurons of the cerebellar nucleus dentatus and nucleus interpositus in cats] Neirofiziologiia. 1980;12:93–96. - PubMed
-
- Bakker DA, Richmond FJ, Abrahams VC, Courville J. Patterns of primary afferent termination in the external cuneate nucleus from cervical axial muscles in the cat. J Comp Neurol. 1985;241:467–479. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
