Ciliary neurotrophic factor receptor regulation of adult forebrain neurogenesis
- PMID: 23325260
- PMCID: PMC3711540
- DOI: 10.1523/JNEUROSCI.3386-12.2013
Ciliary neurotrophic factor receptor regulation of adult forebrain neurogenesis
Abstract
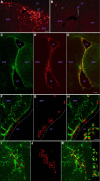
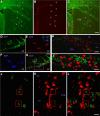
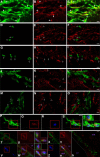
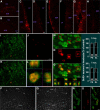
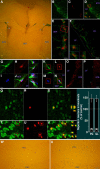
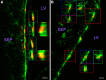
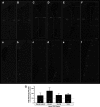
Appropriately targeted manipulation of endogenous neural stem progenitor (NSP) cells may contribute to therapies for trauma, stroke, and neurodegenerative disease. A prerequisite to such therapies is a better understanding of the mechanisms regulating adult NSP cells in vivo. Indirect data suggest that endogenous ciliary neurotrophic factor (CNTF) receptor signaling may inhibit neuronal differentiation of NSP cells. We challenged subventricular zone (SVZ) cells in vivo with low concentrations of CNTF to anatomically characterize cells containing functional CNTF receptors. We found that type B "stem" cells are highly responsive, whereas type C "transit-amplifying" cells and type A neuroblasts are remarkably unresponsive, as are GFAP(+) astrocytes found outside the SVZ. CNTF was identified in a subset of type B cells that label with acute BrdU administration. Disruption of in vivo CNTF receptor signaling in SVZ NSP cells, with a "floxed" CNTF receptor α (CNTFRα) mouse line and a gene construct driving Cre recombinase (Cre) expression in NSP cells, led to increases in SVZ-associated neuroblasts and new olfactory bulb neurons, as well as a neuron subtype-specific, adult-onset increase in olfactory bulb neuron populations. Adult-onset receptor disruption in SVZ NSP cells with a recombinant adeno-associated virus (AAV-Cre) also led to increased neurogenesis. However, the maintenance of type B cell populations was apparently unaffected by the receptor disruption. Together, the data suggest that endogenous CNTF receptor signaling in type B stem cells inhibits adult neurogenesis, and further suggest that the regulation may occur in a neuron subtype-specific manner.
Figures

Similar articles
-
Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice.Exp Neurol. 2003 Oct;183(2):298-310. doi: 10.1016/s0014-4886(03)00129-8. Exp Neurol. 2003. PMID: 14552871
-
The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells.J Neurosci. 2001 Oct 1;21(19):7642-53. doi: 10.1523/JNEUROSCI.21-19-07642.2001. J Neurosci. 2001. PMID: 11567054 Free PMC article.
-
Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats.J Neurosci. 2008 Dec 10;28(50):13368-83. doi: 10.1523/JNEUROSCI.2918-08.2008. J Neurosci. 2008. PMID: 19074010 Free PMC article.
-
Olfactory bulb neurogenesis depending on signaling in the subventricular zone.Cereb Cortex. 2023 Nov 4;33(22):11102-11111. doi: 10.1093/cercor/bhad349. Cereb Cortex. 2023. PMID: 37746807 Review.
-
Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain.Development. 2019 Feb 18;146(4):dev156059. doi: 10.1242/dev.156059. Development. 2019. PMID: 30777863 Free PMC article. Review.
Cited by
-
Genetic Increases in Olfactory Bulb BDNF Do Not Enhance Survival of Adult-Born Granule Cells.Chem Senses. 2020 Jan 1;45(1):3-13. doi: 10.1093/chemse/bjz058. Chem Senses. 2020. PMID: 31562506 Free PMC article.
-
Post-stroke depression and the aging brain.J Mol Psychiatry. 2013 Aug 23;1(1):14. doi: 10.1186/2049-9256-1-14. eCollection 2013. J Mol Psychiatry. 2013. PMID: 25408907 Free PMC article. Review.
-
Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation.Nat Neurosci. 2016 Feb;19(2):243-52. doi: 10.1038/nn.4211. Epub 2016 Jan 11. Nat Neurosci. 2016. PMID: 26752157 Free PMC article.
-
Ciliary Neurotrophic Factor (CNTF) and Its Receptors Signal Regulate Cementoblasts Apoptosis through a Mechanism of ERK1/2 and Caspases Signaling.Int J Mol Sci. 2022 Jul 28;23(15):8335. doi: 10.3390/ijms23158335. Int J Mol Sci. 2022. PMID: 35955469 Free PMC article.
-
Adult Neurogenesis in the Subventricular Zone and Its Regulation After Ischemic Stroke: Implications for Therapeutic Approaches.Transl Stroke Res. 2020 Feb;11(1):60-79. doi: 10.1007/s12975-019-00717-8. Epub 2019 Jul 15. Transl Stroke Res. 2020. PMID: 31309427 Review.
References
-
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. - PubMed
-
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. - PubMed
-
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signaling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
