Selenoprotein N deficiency in mice is associated with abnormal lung development
- PMID: 23325319
- PMCID: PMC3606527
- DOI: 10.1096/fj.12-212688
Selenoprotein N deficiency in mice is associated with abnormal lung development
Abstract
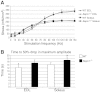
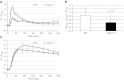
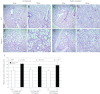
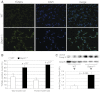
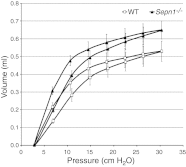
Mutations in the human SEPN1 gene, encoding selenoprotein N (SepN), cause SEPN1-related myopathy (SEPN1-RM) characterized by muscle weakness, spinal rigidity, and respiratory insufficiency. As with other members of the selenoprotein family, selenoprotein N incorporates selenium in the form of selenocysteine (Sec). Most selenoproteins that have been functionally characterized are involved in oxidation-reduction (redox) reactions, with the Sec residue located at their catalytic site. To model SEPN1-RM, we generated a Sepn1-knockout (Sepn1(-/-)) mouse line. Homozygous Sepn1(-/-) mice are fertile, and their weight and lifespan are comparable to wild-type (WT) animals. Under baseline conditions, the muscle histology of Sepn1(-/-) mice remains normal, but subtle core lesions could be detected in skeletal muscle after inducing oxidative stress. Ryanodine receptor (RyR) calcium release channels showed lower sensitivity to caffeine in SepN deficient myofibers, suggesting a possible role of SepN in RyR regulation. SepN deficiency also leads to abnormal lung development characterized by enlarged alveoli, which is associated with decreased tissue elastance and increased quasi-static compliance of Sepn1(-/-) lungs. This finding raises the possibility that the respiratory syndrome observed in patients with SEPN1 mutations may have a primary pulmonary component in addition to the weakness of respiratory muscles.
Figures
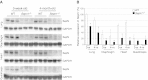
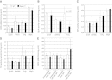

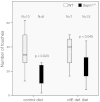
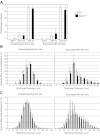
Similar articles
-
Increased muscle stress-sensitivity induced by selenoprotein N inactivation in mouse: a mammalian model for SEPN1-related myopathy.PLoS One. 2011;6(8):e23094. doi: 10.1371/journal.pone.0023094. Epub 2011 Aug 8. PLoS One. 2011. PMID: 21858002 Free PMC article.
-
SEPN1-related myopathy in three patients: novel mutations and diagnostic clues.Eur J Pediatr. 2016 Aug;175(8):1113-8. doi: 10.1007/s00431-015-2685-3. Epub 2016 Jan 16. Eur J Pediatr. 2016. PMID: 26780752
-
Oxidative stress in SEPN1-related myopathy: from pathophysiology to treatment.Ann Neurol. 2009 Jun;65(6):677-86. doi: 10.1002/ana.21644. Ann Neurol. 2009. PMID: 19557870
-
Selenoprotein N in skeletal muscle: from diseases to function.J Mol Med (Berl). 2012 Oct;90(10):1095-107. doi: 10.1007/s00109-012-0896-x. Epub 2012 Apr 14. J Mol Med (Berl). 2012. PMID: 22527882 Review.
-
Calcium and Redox Liaison: A Key Role of Selenoprotein N in Skeletal Muscle.Cells. 2021 May 6;10(5):1116. doi: 10.3390/cells10051116. Cells. 2021. PMID: 34066362 Free PMC article. Review.
Cited by
-
Folliculin regulates cell-cell adhesion, AMPK, and mTORC1 in a cell-type-specific manner in lung-derived cells.Physiol Rep. 2014 Aug 12;2(8):e12107. doi: 10.14814/phy2.12107. Print 2014 Aug 1. Physiol Rep. 2014. PMID: 25121506 Free PMC article.
-
A Chronic Obstructive Pulmonary Disease Susceptibility Gene, FAM13A, Regulates Protein Stability of β-Catenin.Am J Respir Crit Care Med. 2016 Jul 15;194(2):185-97. doi: 10.1164/rccm.201505-0999OC. Am J Respir Crit Care Med. 2016. PMID: 26862784 Free PMC article.
-
Dietary selenium augments sarcoplasmic calcium release and mechanical performance in mice.Nutr Metab (Lond). 2016 Nov 3;13:76. doi: 10.1186/s12986-016-0134-6. eCollection 2016. Nutr Metab (Lond). 2016. PMID: 27822290 Free PMC article.
-
Selenium metabolism and selenoproteins function in brain and encephalopathy.Sci China Life Sci. 2025 Mar;68(3):628-656. doi: 10.1007/s11427-023-2621-7. Epub 2024 Nov 12. Sci China Life Sci. 2025. PMID: 39546178 Review.
-
Endoplasmic Reticulum Oxidative Stress Triggers Tgf-Beta-Dependent Muscle Dysfunction by Accelerating Ascorbic Acid Turnover.Sci Rep. 2017 Jan 20;7:40993. doi: 10.1038/srep40993. Sci Rep. 2017. PMID: 28106121 Free PMC article.
References
-
- Lescure A., Gautheret D., Carbon P., Krol A. (1999) Novel selenoproteins identified in silico and in vivo by using a conserved RNA structural motif. J. Biol. Chem. 274, 38147–38154 - PubMed
-
- Jacob C., Giles G. I., Giles N. M., Sies H. (2003) Sulfur and selenium: the role of oxidation state in protein structure and function. Angew. Chem. Int. Ed. Engl. 42, 4742–4758 - PubMed
-
- Kryukov G. V., Castellano S., Novoselov S. V., Lobanov A. V., Zehtab O., Guigo R., Gladyshev V. N. (2003) Characterization of mammalian selenoproteomes. Science 300, 1439–1443 - PubMed
-
- Behne D., Kyriakopoulos A. (2001) Mammalian selenium-containing proteins. Annu. Rev. Nutr. 21, 453–473 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

