Breaking in: human metapneumovirus fusion and entry
- PMID: 23325326
- PMCID: PMC3564117
- DOI: 10.3390/v5010192
Breaking in: human metapneumovirus fusion and entry
Abstract
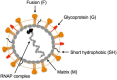
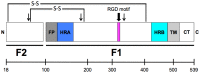
Human metapneumovirus (HMPV) is a leading cause of respiratory infection that causes upper airway and severe lower respiratory tract infections. HMPV infection is initiated by viral surface glycoproteins that attach to cellular receptors and mediate virus membrane fusion with cellular membranes. Most paramyxoviruses use two viral glycoproteins to facilitate virus entry-an attachment protein and a fusion (F) protein. However, membrane fusion for the human paramyxoviruses in the Pneumovirus subfamily, HMPV and respiratory syncytial virus (hRSV), is unique in that the F protein drives fusion in the absence of a separate viral attachment protein. Thus, pneumovirus F proteins can perform the necessary functions for virus entry, i.e., attachment and fusion. In this review, we discuss recent advances in the understanding of how HMPV F mediates both attachment and fusion. We review the requirements for HMPV viral surface glycoproteins during entry and infection, and review the identification of cellular receptors for HMPV F. We also review our current understanding of how HMPV F mediates fusion, concentrating on structural regions of the protein that appear to be critical for membrane fusion activity. Finally, we illuminate key unanswered questions and suggest how further studies can elucidate how this clinically important paramyxovirus fusion protein may have evolved to initiate infection by a unique mechanism.
Figures

Similar articles
-
Roles of the putative integrin-binding motif of the human metapneumovirus fusion (f) protein in cell-cell fusion, viral infectivity, and pathogenesis.J Virol. 2014 Apr;88(8):4338-52. doi: 10.1128/JVI.03491-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478423 Free PMC article.
-
The human metapneumovirus fusion protein mediates entry via an interaction with RGD-binding integrins.J Virol. 2012 Nov;86(22):12148-60. doi: 10.1128/JVI.01133-12. Epub 2012 Aug 29. J Virol. 2012. PMID: 22933271 Free PMC article.
-
[Metapneumovirus expands the understanding of Paramyxovirus cell fusion--a review].Wei Sheng Wu Xue Bao. 2014 Apr 4;54(4):376-82. Wei Sheng Wu Xue Bao. 2014. PMID: 25007649 Review. Chinese.
-
Novel insights into the host cell glycan binding profile of human metapneumovirus.J Virol. 2024 Jun 13;98(6):e0164123. doi: 10.1128/jvi.01641-23. Epub 2024 May 1. J Virol. 2024. PMID: 38690874 Free PMC article.
-
Avian and human metapneumovirus.Ann N Y Acad Sci. 2007 Apr;1102:66-85. doi: 10.1196/annals.1408.005. Ann N Y Acad Sci. 2007. PMID: 17470912 Review.
Cited by
-
Zoonotic Origins of Human Metapneumovirus: A Journey from Birds to Humans.Viruses. 2022 Mar 25;14(4):677. doi: 10.3390/v14040677. Viruses. 2022. PMID: 35458407 Free PMC article. Review.
-
Clinical Insights and Advancements in Human Metapneumovirus Management and Prognosis.Discoveries (Craiova). 2025 Mar 31;13(1):e204. doi: 10.15190/d.2025.3. eCollection 2025 Jan-Mar. Discoveries (Craiova). 2025. PMID: 40351504 Free PMC article. Review.
-
A Candidate Therapeutic Monoclonal Antibody Inhibits Both HRSV and HMPV Replication in Mice.Biomedicines. 2022 Oct 8;10(10):2516. doi: 10.3390/biomedicines10102516. Biomedicines. 2022. PMID: 36289776 Free PMC article.
-
Preclinical evaluation of a live avian metapneumovirus subtype B vaccine strain with cross-protective efficacy in chickens.Poult Sci. 2025 Aug;104(8):105283. doi: 10.1016/j.psj.2025.105283. Epub 2025 May 10. Poult Sci. 2025. PMID: 40398299 Free PMC article.
-
Genome-Wide Analysis of Human Metapneumovirus Evolution.PLoS One. 2016 Apr 5;11(4):e0152962. doi: 10.1371/journal.pone.0152962. eCollection 2016. PLoS One. 2016. PMID: 27046055 Free PMC article.
References
-
- Cook J.K. Avian pneumovirus infections of turkeys and chickens. Vet. J. 2000;160:118–125. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

