Respiratory syncytial virus entry inhibitors targeting the F protein
- PMID: 23325327
- PMCID: PMC3564118
- DOI: 10.3390/v5010211
Respiratory syncytial virus entry inhibitors targeting the F protein
Abstract
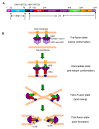
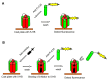
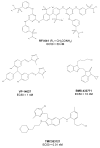
Human respiratory syncytial virus (RSV) is the main viral cause of respiratory tract infection in infants as well as some elderly and high-risk adults with chronic pulmonary disease and the severely immunocompromised. So far, no specific anti-RSV therapeutics or effective anti-RSV vaccines have been reported. Only one humanized monoclonal antibody, Palivizumab, has been approved for use in high-risk infants to prevent RSV infection. Ribavirin is the only drug licensed for therapy of RSV infection, but its clinical use is limited by its nonspecific anti-RSV activity, toxic effect, and relatively high cost. Therefore, development of novel effective anti-RSV therapeutics is urgently needed. The RSV envelope glycoprotein F plays an important role in RSV fusion with, and entry into, the host cell and, consequently, serves as an attractive target for developing RSV entry inhibitors. This article reviews advances made in studies of the structure and function of the F protein and the development of RSV entry inhibitors targeting it.
Figures
References
-
- Morris J.A., Blount R.E., Savage R.E. Recovery of cytopathic agent from chimpanzees with coryza. Proc. Soc. Exp. Biol. Med. 1956;92:544–550. - PubMed
-
- Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005;352:1749–1759. - PubMed
-
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O'Brien K.L., Roca A., Wright P.F., Bruce N., et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

