Physiology of SLC12 transporters: lessons from inherited human genetic mutations and genetically engineered mouse knockouts
- PMID: 23325410
- PMCID: PMC3625803
- DOI: 10.1152/ajpcell.00350.2012
Physiology of SLC12 transporters: lessons from inherited human genetic mutations and genetically engineered mouse knockouts
Abstract
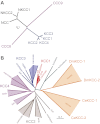
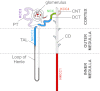
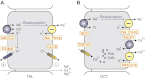
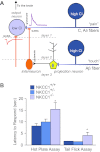
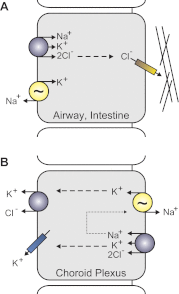
Among the over 300 members of the solute carrier (SLC) group of integral plasma membrane transport proteins are the nine electroneutral cation-chloride cotransporters belonging to the SLC12 gene family. Seven of these transporters have been functionally described as coupling the electrically silent movement of chloride with sodium and/or potassium. Although in silico analysis has identified two additional SLC12 family members, no physiological role has been ascribed to the proteins encoded by either the SLC12A8 or the SLC12A9 genes. Evolutionary conservation of this gene family from protists to humans confirms their importance. A wealth of physiological, immunohistochemical, and biochemical studies have revealed a great deal of information regarding the importance of this gene family to human health and disease. The sequencing of the human genome has provided investigators with the capability to link several human diseases with mutations in the genes encoding these plasma membrane proteins. The availability of bacterial artificial chromosomes, recombination engineering techniques, and the mouse genome sequence has simplified the creation of targeting constructs to manipulate the expression/function of these cation-chloride cotransporters in the mouse in an attempt to recapitulate some of these human pathologies. This review will summarize the three human disorders that have been linked to the mutation/dysfunction of the Na-Cl, Na-K-2Cl, and K-Cl cotransporters (i.e., Bartter's, Gitleman's, and Andermann's syndromes), examine some additional pathologies arising from genetically modified mouse models of these cotransporters including deafness, blood pressure, hyperexcitability, and epithelial transport deficit phenotypes.
Figures



Similar articles
-
Molecular physiology of cation-coupled Cl- cotransport: the SLC12 family.Pflugers Arch. 2004 Feb;447(5):580-93. doi: 10.1007/s00424-003-1066-3. Epub 2003 May 9. Pflugers Arch. 2004. PMID: 12739168 Review.
-
[Pathophysiological aspects of K+: Cl- cotransporters].Rev Invest Clin. 2014 Mar-Apr;66(2):173-80. Rev Invest Clin. 2014. PMID: 24960328 Review. Spanish.
-
From Fish Physiology to Human Disease: The Discovery of the NCC, NKCC2, and the Cation-Coupled Chloride Cotransporters.Kidney360. 2024 Jan 1;5(1):133-141. doi: 10.34067/KID.0000000000000307. Epub 2023 Nov 16. Kidney360. 2024. PMID: 37968800 Free PMC article.
-
Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters.Physiol Rev. 2005 Apr;85(2):423-93. doi: 10.1152/physrev.00011.2004. Physiol Rev. 2005. PMID: 15788703 Review.
-
The SLC12 family of electroneutral cation-coupled chloride cotransporters.Mol Aspects Med. 2013 Apr-Jun;34(2-3):288-98. doi: 10.1016/j.mam.2012.05.002. Mol Aspects Med. 2013. PMID: 23506871 Review.
Cited by
-
Chloride-dependent conformational changes in the GlyT1 glycine transporter.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2017431118. doi: 10.1073/pnas.2017431118. Proc Natl Acad Sci U S A. 2021. PMID: 33658361 Free PMC article.
-
Molecular and evolutionary insights into the structural organization of cation chloride cotransporters.Front Cell Neurosci. 2015 Jan 21;8:470. doi: 10.3389/fncel.2014.00470. eCollection 2014. Front Cell Neurosci. 2015. PMID: 25653592 Free PMC article. Review.
-
NKCC1 and KCC2: Structural insights into phospho-regulation.Front Mol Neurosci. 2022 Jul 22;15:964488. doi: 10.3389/fnmol.2022.964488. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35935337 Free PMC article. Review.
-
OSR1 regulates a subset of inward rectifier potassium channels via a binding motif variant.Proc Natl Acad Sci U S A. 2018 Apr 10;115(15):3840-3845. doi: 10.1073/pnas.1802339115. Epub 2018 Mar 26. Proc Natl Acad Sci U S A. 2018. PMID: 29581290 Free PMC article.
-
A genome-wide association study in a large F2-cross of laying hens reveals novel genomic regions associated with feather pecking and aggressive pecking behavior.Genet Sel Evol. 2017 Feb 3;49(1):18. doi: 10.1186/s12711-017-0287-4. Genet Sel Evol. 2017. PMID: 28158968 Free PMC article.
References
-
- Adragna NC, Chen Y, Delpire E, Lauf PK, Morris M. Hypertension in K-Cl cotransporter-3 knockout mice. Adv Exp Med Biol 559: 379–385, 2004 - PubMed
-
- Alvarez-Leefmans FJ, Leon-Olea M, Mendoza-Sotelo J, Alvarez FJ, Anton B, Garduno R. Immunolocalization of the Na(+)-K(+)-2Cl(-) cotransporter in peripheral nervous tissue of vertebrates. Neuroscience 104: 569–582, 2002 - PubMed
-
- Andermann F, Andermann E, Joubert M, Karpati G, Carpenter S, Melancon D. Familial agenesis of the corpus callosum with anterior horn cell disease: a syndrome of mental retardation, areflexia and paraparesis. Trans Am Neurol Assoc 97: 242–244, 1972
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

