Shank2 contributes to the apical retention and intracellular redistribution of NaPiIIa in OK cells
- PMID: 23325414
- PMCID: PMC3674434
- DOI: 10.1152/ajpcell.00189.2012
Shank2 contributes to the apical retention and intracellular redistribution of NaPiIIa in OK cells
Abstract
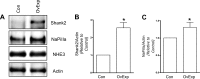
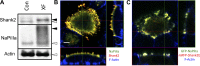
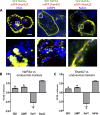
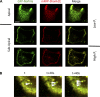
In renal proximal tubule (PT) cells, sodium-phosphate cotransporter IIa (NaPiIIa) is normally concentrated within the apical membrane where it reabsorbs ∼70% of luminal phosphate (Pi). NaPiIIa activity is acutely regulated by moderating its abundance within the apical membrane. Under low-Pi conditions, NaPiIIa is retained within the apical membrane. Under high-Pi conditions, NaPiIIa is retrieved from the apical membrane and trafficked to the lysosomes for degradation. The present study investigates the role of Shank2 in regulating the distribution of NaPiIIa. In opossum kidney cells, a PT cell model, knockdown of Shank2 in cells maintained in low-Pi media resulted in a marked decrease in NaPiIIa abundance. After being transferred into high-Pi media, live-cell imaging showed that mRFP-Shank2E and GFP-NaPiIIa underwent endocytosis and trafficked together through the subapical domain. Fluorescence cross-correlation spectroscopy demonstrated that GFP-NaPiIIa and mRFP-Shank2 have indistinguishable diffusion coefficients and migrated through the subapical domain in temporal synchrony. Raster image cross-correlation spectroscopy demonstrated these two proteins course through the subapical domain in temporal-spatial synchrony. In the microvilli of cells under low-Pi conditions and in the subapical domain of cells under high-Pi conditions, fluorescence lifetime imaging microscopy-Forster resonance energy transfer analysis of Cer-NaPiIIa and EYFP-Shank2E found these fluors reside within 10 nm of each other. Demonstrating a complexity of functions, in cells maintained under low-Pi conditions, Shank2 plays an essential role in the apical retention of NaPiIIa while under high-Pi conditions Shank2 remains associated with NaPiIIa and escorts NaPiIIa through the cell interior.
Figures




Similar articles
-
Shank2 redistributes with NaPilla during regulated endocytosis.Am J Physiol Cell Physiol. 2010 Dec;299(6):C1324-34. doi: 10.1152/ajpcell.00183.2010. Epub 2010 Sep 1. Am J Physiol Cell Physiol. 2010. PMID: 20810910 Free PMC article.
-
Shank2E binds NaP(i) cotransporter at the apical membrane of proximal tubule cells.Am J Physiol Cell Physiol. 2005 Oct;289(4):C1042-51. doi: 10.1152/ajpcell.00568.2004. Epub 2005 May 25. Am J Physiol Cell Physiol. 2005. PMID: 15917299
-
Interaction of MAP17 with NHERF3/4 induces translocation of the renal Na/Pi IIa transporter to the trans-Golgi.Am J Physiol Renal Physiol. 2007 Jan;292(1):F230-42. doi: 10.1152/ajprenal.00075.2006. Epub 2006 Aug 22. Am J Physiol Renal Physiol. 2007. PMID: 16926447
-
Molecular mechanisms in proximal tubular and small intestinal phosphate reabsorption (plenary lecture).Mol Membr Biol. 2001 Jan-Mar;18(1):3-11. doi: 10.1080/09687680010019357. Mol Membr Biol. 2001. PMID: 11396609 Review.
-
Regulation of Na/Pi transporter in the proximal tubule.Annu Rev Physiol. 2003;65:531-42. doi: 10.1146/annurev.physiol.65.042902.092424. Epub 2002 May 1. Annu Rev Physiol. 2003. PMID: 12517995 Review.
Cited by
-
Mapping diffusion in a living cell via the phasor approach.Biophys J. 2014 Dec 16;107(12):2775-2785. doi: 10.1016/j.bpj.2014.08.041. Biophys J. 2014. PMID: 25517145 Free PMC article.
-
Phasor Analysis of Local ICS Detects Heterogeneity in Size and Number of Intracellular Vesicles.Biophys J. 2016 Aug 9;111(3):619-629. doi: 10.1016/j.bpj.2016.06.029. Biophys J. 2016. PMID: 27508445 Free PMC article.
-
Advances in fluorescence microscopy techniques to study kidney function.Nat Rev Nephrol. 2021 Feb;17(2):128-144. doi: 10.1038/s41581-020-00337-8. Epub 2020 Sep 18. Nat Rev Nephrol. 2021. PMID: 32948857 Review.
-
Visualizing the regulation of SLC34 proteins at the apical membrane.Pflugers Arch. 2019 Apr;471(4):533-542. doi: 10.1007/s00424-018-02249-w. Epub 2019 Jan 6. Pflugers Arch. 2019. PMID: 30613865 Free PMC article. Review.
-
Renal phosphate transporters.Curr Opin Nephrol Hypertens. 2014 Sep;23(5):502-6. doi: 10.1097/MNH.0000000000000053. Curr Opin Nephrol Hypertens. 2014. PMID: 25028980 Free PMC article. Review.
References
-
- Bastepe M, Juppner H. Inherited hypophosphatemic disorders in children and the evolving mechanisms of phosphate regulation. Rev Endocr Metab Disord 9: 171–180, 2008. - PubMed
-
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Reiss A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet 42: 489–491, 2010. - PubMed
-
- Capuano P, Bacic D, Stange G, Hernando N, Kaissling B, Pal R, Kocher O, Biber J, Wagner CA, Murer H. Expression and regulation of the renal Na/Pi cotransporter NaPi-IIa in a mouse model deficient for the PDZ protein, PDZK1. Pflügers Arch 449: 392–402, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

