Safety of fluconazole in paediatrics: a systematic review
- PMID: 23325436
- PMCID: PMC3651820
- DOI: 10.1007/s00228-012-1468-2
Safety of fluconazole in paediatrics: a systematic review
Abstract
Purpose: To determine the safety of fluconazole in neonates and other paediatric age groups by identifying adverse events (AEs) and drug interactions associated with treatment.
Methods: A search of EMBASE (1950-January 2012), MEDLINE (1946-January 2012), the Cochrane database for systematic reviews and the Cumulative Index to Nursing and Allied Health Literature (1982-2012) for any clinical study about fluconazole use that involved at least one paediatric patient (≤17 years) was performed. Only articles with sufficient quality of safety reporting after patients' exposure to fluconazole were included.
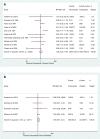
Results: We identified 90 articles, reporting on 4,209 patients, which met our inclusion criteria. In total, 794 AEs from 35 studies were recorded, with hepatotoxicity accounting for 378 (47.6 %) of all AEs. When fluconazole was compared with placebo and other antifungals, the relative risk (RR) of hepatotoxicity was not statistically different [RR 1.36, 95 % confidence interval (CI) 0.87-2.14, P = 0.175 and RR 1.43, 95 % CI 0.67-3.03, P = 0.352, respectively]. Complete resolution of hepatoxicity was achieved by 84 % of patients with follow-up available. There was no statistical difference in the risk of gastrointestinal events of fluconazole compared with placebo and other antifungals (RR 0.81, 95 % CI 0.12-5.60, P = 0.831 and RR 1.23, 95 %CI 0.87-1.71, P = 0.235, respectively). There were 41 drug withdrawals, 17 (42 %) of which were due to elevated liver enzymes. Five reports of drug interactions occurred in children.
Conclusion: Fluconazole is relatively safe for paediatric patients. Hepatotoxicity and gastrointestinal toxicity are the most common adverse events. It is important to be aware that drug interactions with fluconazole can result in significant toxicity.
Figures

References
Supplementary references
-
- Bunin N. Oral fluconazole for treatment of disseminated fungal infection. Pediatr Infect Dis J. 1989;8(1):62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

