Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells
- PMID: 23325555
- PMCID: PMC3633649
- DOI: 10.1002/hep.26237
Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells
Abstract
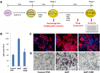
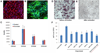
Patient-specific induced pluripotent stem cells (iPSCs) represent a potential source for developing novel drug and cell therapies. Although increasing numbers of disease-specific iPSCs have been generated, there has been limited progress in iPSC-based drug screening/discovery for liver diseases, and the low gene-targeting efficiency in human iPSCs warrants further improvement. Using iPSC lines from patients with alpha-1 antitrypsin (AAT) deficiency, for which there is currently no drug or gene therapy available, we established a platform to discover new drug candidates and correct disease-causing mutation with a high efficiency. A high-throughput format screening assay, based on our hepatic differentiation protocol, was implemented to facilitate automated quantification of cellular AAT accumulation using a 96-well immunofluorescence reader. To expedite the eventual application of lead compounds to patients, we conducted drug screening utilizing our established library of clinical compounds (the Johns Hopkins Drug Library) with extensive safety profiles. Through a blind large-scale drug screening, five clinical drugs were identified to reduce AAT accumulation in diverse patient iPSC-derived hepatocyte-like cells. In addition, using the recently developed transcription activator-like effector nuclease technology, we achieved high gene-targeting efficiency in AAT-deficiency patient iPSCs with 25%-33% of the clones demonstrating simultaneous targeting at both diseased alleles. The hepatocyte-like cells derived from the gene-corrected iPSCs were functional without the mutant AAT accumulation. This highly efficient and cost-effective targeting technology will broadly benefit both basic and translational applications.
Conclusions: Our results demonstrated the feasibility of effective large-scale drug screening using an iPSC-based disease model and highly robust gene targeting in human iPSCs, both of which are critical for translating the iPSC technology into novel therapies for untreatable diseases.
Copyright © 2013 American Association for the Study of Liver Diseases.
Figures


Similar articles
-
Sustained knockdown of a disease-causing gene in patient-specific induced pluripotent stem cells using lentiviral vector-based gene therapy.Stem Cells Transl Med. 2013 Sep;2(9):641-54. doi: 10.5966/sctm.2013-0017. Epub 2013 Aug 7. Stem Cells Transl Med. 2013. PMID: 23926210 Free PMC article.
-
Adenine base editing reduces misfolded protein accumulation and toxicity in alpha-1 antitrypsin deficient patient iPSC-hepatocytes.Mol Ther. 2021 Nov 3;29(11):3219-3229. doi: 10.1016/j.ymthe.2021.06.021. Epub 2021 Jul 2. Mol Ther. 2021. PMID: 34217893 Free PMC article.
-
Emergence of a stage-dependent human liver disease signature with directed differentiation of alpha-1 antitrypsin-deficient iPS cells.Stem Cell Reports. 2015 May 12;4(5):873-85. doi: 10.1016/j.stemcr.2015.02.021. Epub 2015 Apr 2. Stem Cell Reports. 2015. PMID: 25843048 Free PMC article.
-
Potential applications of induced pluripotent stem cells (iPSCs) in hepatology research.Curr Stem Cell Res Ther. 2015;10(3):208-15. doi: 10.2174/1574888x10666150120105946. Curr Stem Cell Res Ther. 2015. PMID: 25599714 Review.
-
Thinking outside the liver: induced pluripotent stem cells for hepatic applications.World J Gastroenterol. 2013 Jun 14;19(22):3385-96. doi: 10.3748/wjg.v19.i22.3385. World J Gastroenterol. 2013. PMID: 23801830 Free PMC article. Review.
Cited by
-
Efficient and Controlled Generation of 2D and 3D Bile Duct Tissue from Human Pluripotent Stem Cell-Derived Spheroids.Stem Cell Rev Rep. 2016 Aug;12(4):500-8. doi: 10.1007/s12015-016-9657-5. Stem Cell Rev Rep. 2016. PMID: 27138846 Free PMC article.
-
Mapping the Cell-Surface N-Glycoproteome of Human Hepatocytes Reveals Markers for Selecting a Homogeneous Population of iPSC-Derived Hepatocytes.Stem Cell Reports. 2016 Sep 13;7(3):543-556. doi: 10.1016/j.stemcr.2016.07.016. Epub 2016 Aug 25. Stem Cell Reports. 2016. PMID: 27569060 Free PMC article.
-
Novel approaches to liver disease diagnosis and modeling.Transl Gastroenterol Hepatol. 2021 Apr 5;6:19. doi: 10.21037/tgh-20-109. eCollection 2021. Transl Gastroenterol Hepatol. 2021. PMID: 33824923 Free PMC article. Review.
-
Advancements in Disease Modeling and Drug Discovery Using iPSC-Derived Hepatocyte-like Cells.Genes (Basel). 2022 Mar 24;13(4):573. doi: 10.3390/genes13040573. Genes (Basel). 2022. PMID: 35456379 Free PMC article. Review.
-
Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9.Stem Cell Reports. 2015 Jan 13;4(1):143-154. doi: 10.1016/j.stemcr.2014.10.013. Epub 2014 Nov 26. Stem Cell Reports. 2015. PMID: 25434822 Free PMC article.
References
-
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. - PubMed
-
- Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–968. - PubMed
-
- Mayr LM, Fuerst P. The future of high-throughput screening. J Biomol Screen. 2008;13:443–448. - PubMed
-
- Wang J, Skolnik S. Recent advances in physicochemical and ADMET profiling in drug discovery. Chem Biodivers. 2009;6:1887–1899. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
