Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells
- PMID: 23325555
- PMCID: PMC3633649
- DOI: 10.1002/hep.26237
Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells
Abstract
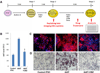
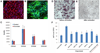
Patient-specific induced pluripotent stem cells (iPSCs) represent a potential source for developing novel drug and cell therapies. Although increasing numbers of disease-specific iPSCs have been generated, there has been limited progress in iPSC-based drug screening/discovery for liver diseases, and the low gene-targeting efficiency in human iPSCs warrants further improvement. Using iPSC lines from patients with alpha-1 antitrypsin (AAT) deficiency, for which there is currently no drug or gene therapy available, we established a platform to discover new drug candidates and correct disease-causing mutation with a high efficiency. A high-throughput format screening assay, based on our hepatic differentiation protocol, was implemented to facilitate automated quantification of cellular AAT accumulation using a 96-well immunofluorescence reader. To expedite the eventual application of lead compounds to patients, we conducted drug screening utilizing our established library of clinical compounds (the Johns Hopkins Drug Library) with extensive safety profiles. Through a blind large-scale drug screening, five clinical drugs were identified to reduce AAT accumulation in diverse patient iPSC-derived hepatocyte-like cells. In addition, using the recently developed transcription activator-like effector nuclease technology, we achieved high gene-targeting efficiency in AAT-deficiency patient iPSCs with 25%-33% of the clones demonstrating simultaneous targeting at both diseased alleles. The hepatocyte-like cells derived from the gene-corrected iPSCs were functional without the mutant AAT accumulation. This highly efficient and cost-effective targeting technology will broadly benefit both basic and translational applications.
Conclusions: Our results demonstrated the feasibility of effective large-scale drug screening using an iPSC-based disease model and highly robust gene targeting in human iPSCs, both of which are critical for translating the iPSC technology into novel therapies for untreatable diseases.
Copyright © 2013 American Association for the Study of Liver Diseases.
Figures


References
-
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. - PubMed
-
- Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–968. - PubMed
-
- Mayr LM, Fuerst P. The future of high-throughput screening. J Biomol Screen. 2008;13:443–448. - PubMed
-
- Wang J, Skolnik S. Recent advances in physicochemical and ADMET profiling in drug discovery. Chem Biodivers. 2009;6:1887–1899. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
