Adaptation to the interferon-induced antiviral state by human and simian immunodeficiency viruses
- PMID: 23325684
- PMCID: PMC3592164
- DOI: 10.1128/JVI.03219-12
Adaptation to the interferon-induced antiviral state by human and simian immunodeficiency viruses
Erratum in
-
Correction for Bitzegeio et al., Adaptation to the Interferon-Induced Antiviral State by Human and Simian Immunodeficiency Viruses.J Virol. 2015 Oct;89(19):10133. doi: 10.1128/JVI.01711-15. J Virol. 2015. PMID: 26330490 Free PMC article. No abstract available.
Abstract
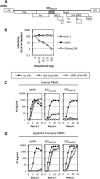
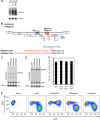
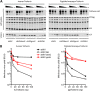
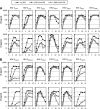
The production of type I interferon (IFN) is an early host response to different infectious agents leading to the induction of hundreds of IFN-stimulated genes (ISGs). The roles of many ISGs in host defense are unknown, but their expression results in the induction of an "antiviral state" that inhibits the replication of many viruses. Here we show that prototype primate lentiviruses human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus of macaques (SIV(MAC) and SIV(MNE)) can replicate in lymphocytes from their usual hosts (humans and macaques, respectively), even when an antiviral state is induced by IFN-α treatment. In contrast, HIV-1 and SIV(MAC)/SIV(MNE) replication was hypersensitive to IFN-α in lymphocytes from unnatural hosts, indicating that the antiviral state can effectively curtail the replication of primate lentiviruses in hosts to which they are not adapted. Most of the members of a panel of naturally occurring HIV-1 and HIV-2 strains behaved like prototype strains and were comparatively insensitive to IFN-α in human lymphocytes. Using chimeric viruses engineered to overcome restriction factors whose antiretroviral specificities vary in a species-dependent manner, we demonstrate that differential HIV-1 and SIV(MAC) sensitivities to IFN-α in lymphocytes from humans and macaques could not be ascribed to TRIM5, APOBEC3, tetherin, or SAMHD1. Single-cycle infection experiments indicated that at least part of this species-specific, IFN-α-induced restriction of primate lentivirus replication occurs early in the retroviral life cycle. Overall, these studies indicate the existence of undiscovered, IFN-α-inducible antiretroviral factors whose spectrum of activity varies in a species-dependent manner and to which at least some HIV/SIV strains have become adapted in their usual hosts.
Figures




Comment in
-
Findings of research misconduct.NIH Guide Grants Contracts (Bethesda). 2015 Jul 31:NOT-OD-15-131. NIH Guide Grants Contracts (Bethesda). 2015. PMID: 26241998 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2015 Jul 23;80(141):43784. Fed Regist. 2015. PMID: 27737258 Free PMC article. No abstract available.
References
-
- Gaines H, von Sydow MA, von Stedingk LV, Biberfeld G, Bottiger B, Hansson LO, Lundbergh P, Sonnerborg AB, Wasserman J, Strannegaard OO. 1990. Immunological changes in primary HIV-1 infection. AIDS 4:995–999 - PubMed
-
- Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119:3544–3555 - PMC - PubMed
-
- Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. 2008. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood 112:4598–4608 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

