Reevaluation of the requirement for TIP47 in human immunodeficiency virus type 1 envelope glycoprotein incorporation
- PMID: 23325685
- PMCID: PMC3592152
- DOI: 10.1128/JVI.03299-12
Reevaluation of the requirement for TIP47 in human immunodeficiency virus type 1 envelope glycoprotein incorporation
Abstract
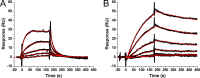
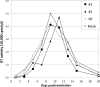
Incorporation of the human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins into assembling particles is crucial for virion infectivity. Genetic and biochemical data indicate that the matrix (MA) domain of Gag and the cytoplasmic tail of the transmembrane glycoprotein gp41 play an important role in coordinating Env incorporation; however, the molecular mechanism and possible role of host factors in this process remain to be defined. Recent studies suggested that Env incorporation is mediated by interactions between matrix and tail-interacting protein of 47 kDa (TIP47; also known as perilipin-3 and mannose-6-phosphate receptor-binding protein 1), a member of the perilipin, adipophilin, TIP47 (PAT) family of proteins implicated in protein sorting and lipid droplet biogenesis. We have confirmed by nuclear magnetic resonance spectroscopy titration experiments and surface plasmon resonance that MA binds TIP47. We also reevaluated the role of TIP47 in HIV-1 Env incorporation in HeLa cells and in the Jurkat T-cell line. In HeLa cells, TIP47 overexpression or RNA interference (RNAi)-mediated depletion had no significant effect on HIV-1 Env incorporation, virus release, or particle infectivity. Similarly, depletion of TIP47 in Jurkat cells did not impair HIV-1 Env incorporation, virus release, infectivity, or replication. Our results thus do not support a role for TIP47 in HIV-1 Env incorporation or virion infectivity.
Figures




Similar articles
-
Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions.Proc Natl Acad Sci U S A. 2006 Oct 3;103(40):14947-52. doi: 10.1073/pnas.0602941103. Epub 2006 Sep 26. Proc Natl Acad Sci U S A. 2006. PMID: 17003132 Free PMC article.
-
Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity.J Virol. 2003 Jun;77(12):6931-45. doi: 10.1128/jvi.77.12.6931-6945.2003. J Virol. 2003. PMID: 12768012 Free PMC article.
-
TIP47 is required for the production of infectious HIV-1 particles from primary macrophages.Traffic. 2010 Apr;11(4):455-67. doi: 10.1111/j.1600-0854.2010.01036.x. Epub 2010 Jan 11. Traffic. 2010. PMID: 20070608
-
The role of matrix in HIV-1 envelope glycoprotein incorporation.Trends Microbiol. 2014 Jul;22(7):372-8. doi: 10.1016/j.tim.2014.04.012. Epub 2014 Jun 2. Trends Microbiol. 2014. PMID: 24933691 Free PMC article. Review.
-
HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation.J Mol Biol. 2011 Jul 22;410(4):582-608. doi: 10.1016/j.jmb.2011.04.042. J Mol Biol. 2011. PMID: 21762802 Free PMC article. Review.
Cited by
-
A single G10T polymorphism in HIV-1 subtype C Gag-SP1 regulates sensitivity to maturation inhibitors.Retrovirology. 2021 Apr 9;18(1):9. doi: 10.1186/s12977-021-00553-5. Retrovirology. 2021. PMID: 33836787 Free PMC article.
-
A tyrosine-based motif in the HIV-1 envelope glycoprotein tail mediates cell-type- and Rab11-FIP1C-dependent incorporation into virions.Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):7575-80. doi: 10.1073/pnas.1504174112. Epub 2015 Jun 1. Proc Natl Acad Sci U S A. 2015. PMID: 26034275 Free PMC article.
-
Viral and Host Factors Regulating HIV-1 Envelope Protein Trafficking and Particle Incorporation.Viruses. 2022 Aug 5;14(8):1729. doi: 10.3390/v14081729. Viruses. 2022. PMID: 36016351 Free PMC article. Review.
-
HIV-1 Hijacking of Host ATPases and GTPases That Control Protein Trafficking.Front Cell Dev Biol. 2021 Jul 8;9:622610. doi: 10.3389/fcell.2021.622610. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34307340 Free PMC article. Review.
-
Retromer regulates HIV-1 envelope glycoprotein trafficking and incorporation into virions.PLoS Pathog. 2014 Nov 13;10(10):e1004518. doi: 10.1371/journal.ppat.1004518. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25393110 Free PMC article.
References
-
- Sundquist WI, Krausslich HG. 2012. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2:a006924 doi:10.1101/cshperspect.a006924 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

