A key interaction between the alphavirus envelope proteins responsible for initial dimer dissociation during fusion
- PMID: 23325694
- PMCID: PMC3624238
- DOI: 10.1128/JVI.03310-12
A key interaction between the alphavirus envelope proteins responsible for initial dimer dissociation during fusion
Abstract
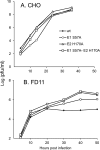
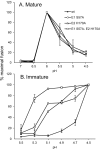
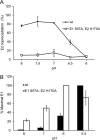
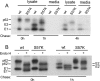
Alphaviruses such as Semliki Forest virus (SFV) are enveloped viruses whose surface is covered by an organized lattice composed of trimers of E2-E1 heterodimers. The E1 envelope protein, a class II fusion protein, contains the hydrophobic fusion loop and refolds to drive virus fusion with the endosome membrane. The E2 protein is synthesized as a precursor p62, whose processing by furin primes the heterodimer for dissociation during virus entry. Dissociation of the E2-E1 heterodimer is an essential step during low-pH-triggered fusion, while the dissociation of the immature p62-E1 dimer is relatively pH resistant. Previous structural studies described an "acid-sensitive region" in E2 that becomes disordered at low pH. Within this region, the conserved E2 H170 is in position to form a hydrogen bond with the underlying E1 S57. Here we experimentally tested the role of this interaction in regulating dimer dissociation in mature and immature virus. Alanine substitutions of E1 S57 and E2 H170 destabilized the heterodimer and produced a higher pH threshold for exposure of the E1 fusion loop and for fusion of the immature virus. E1 S57K or S57D mutations were lethal and caused transport and assembly defects that were partially abrogated by neutralization of the exocytic pathway. The lethal phenotype of E1 S57K was rescued by second-site mutations at E2 H170/M171. Together, our results define a key role for the E1 S57-E2 H170 interaction in dimer stability and the pH dependence of fusion and provide evidence for stepwise dissociation of the E2-E1 dimer at low pH.
Figures



Similar articles
-
An Alphavirus E2 Membrane-Proximal Domain Promotes Envelope Protein Lateral Interactions and Virus Budding.mBio. 2017 Nov 7;8(6):e01564-17. doi: 10.1128/mBio.01564-17. mBio. 2017. PMID: 29114027 Free PMC article.
-
The role of E3 in pH protection during alphavirus assembly and exit.J Virol. 2013 Sep;87(18):10255-62. doi: 10.1128/JVI.01507-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864626 Free PMC article.
-
Role of conserved histidine residues in the low-pH dependence of the Semliki Forest virus fusion protein.J Virol. 2009 May;83(9):4670-7. doi: 10.1128/JVI.02646-08. Epub 2009 Feb 25. J Virol. 2009. PMID: 19244325 Free PMC article.
-
Assembly and entry mechanisms of Semliki Forest virus.Arch Virol Suppl. 1994;9:329-38. doi: 10.1007/978-3-7091-9326-6_33. Arch Virol Suppl. 1994. PMID: 8032265 Review.
-
The Envelope Proteins of the Bunyavirales.Adv Virus Res. 2017;98:83-118. doi: 10.1016/bs.aivir.2017.02.002. Epub 2017 Apr 8. Adv Virus Res. 2017. PMID: 28433053 Review.
Cited by
-
Residue-level resolution of alphavirus envelope protein interactions in pH-dependent fusion.Proc Natl Acad Sci U S A. 2015 Feb 17;112(7):2034-9. doi: 10.1073/pnas.1414190112. Epub 2015 Feb 2. Proc Natl Acad Sci U S A. 2015. PMID: 25646410 Free PMC article.
-
Two Conserved Phenylalanine Residues in the E1 Fusion Loop of Alphaviruses Are Essential for Viral Infectivity.J Virol. 2022 May 11;96(9):e0006422. doi: 10.1128/jvi.00064-22. Epub 2022 Apr 13. J Virol. 2022. PMID: 35416719 Free PMC article.
-
Human monoclonal antibodies against Ross River virus target epitopes within the E2 protein and protect against disease.PLoS Pathog. 2020 May 4;16(5):e1008517. doi: 10.1371/journal.ppat.1008517. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32365139 Free PMC article.
-
An Alphavirus E2 Membrane-Proximal Domain Promotes Envelope Protein Lateral Interactions and Virus Budding.mBio. 2017 Nov 7;8(6):e01564-17. doi: 10.1128/mBio.01564-17. mBio. 2017. PMID: 29114027 Free PMC article.
-
Interactions involved in pH protection of the alphavirus fusion protein.Virology. 2015 Dec;486:173-9. doi: 10.1016/j.virol.2015.08.028. Epub 2015 Oct 2. Virology. 2015. PMID: 26433749 Free PMC article.
References
-
- Kuhn RJ. 2007. Togaviridae: the viruses and their replication, p 1001–1022 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed, vol 1 Lippincott, Williams and Wilkins, Philadelphia, PA
-
- Enserink M. 2007. Infectious diseases. Chikungunya: no longer a third world disease. Science 318:1860–1861 - PubMed
-
- Schwartz O, Albert ML. 2010. Biology and pathogenesis of Chikungunya virus. Nat. Rev. Microbiol. 8:491–500 - PubMed
-
- Mancini EJ, Clarke M, Gowen BE, Rutten T, Fuller SD. 2000. Cryo-electron microscopy reveals the functional organization of an enveloped virus, Semliki forest virus. Mol. Cell 5:255–266 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

