Positive bias in mycophenolic acid concentrations determined by the CEDIA assay compared to HPLC-UV method: is CEDIA assay suitable for therapeutic drug monitoring of mycophenolic acid?
- PMID: 23325745
- PMCID: PMC6807549
- DOI: 10.1002/jcla.21565
Positive bias in mycophenolic acid concentrations determined by the CEDIA assay compared to HPLC-UV method: is CEDIA assay suitable for therapeutic drug monitoring of mycophenolic acid?
Abstract
Background: Both immunoassays and chromatographic methods are available for therapeutic drug monitoring of mycophenolic acid (MPA), an immunosuppressant. We studied the suitability of cloned enzyme donor immunoassay (CEDIA) assay for routine monitoring of MPA by comparing values obtained by the CEDIA assay with corresponding values obtained by using a high-performance liquid chromatography combined with ultraviolet detection (HPLC-UV) method.
Methods: We compared MPA concentrations obtained by a reference HPLC-UV method and CEDIA assay on Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, IN) using 60 patient specimens (18 liver transplant recipient and 42 kidney transplant recipients).
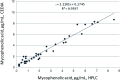
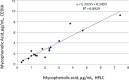
Results: When MPA concentrations in all 60 transplant recipients obtained by the HPLC-UV (x-axis) method were compared with corresponding values obtained by the CEDIA method (y-axis), the following regression equation was obtained: y = 1.1558x + 0.2876 (r = 0.97). Interestingly, much lower bias was observed in 42 renal transplant recipients as revealed by the following regression equation; y = 1.1181x + 0.2745 (r = 0.98). However, more significant positive bias was observed in 18 liver transplant recipients as following regression equation as observed: y = 1.3337x + 0.1493 (r = 0.94).
Conclusions: We conclude that MPA concentrations determined by the CEDIA assay showed significant positive bias compared to HPLC-UV method. Therefore, caution must be exercised in interpreting therapeutic drug monitoring result of MPA if CEDIA assay is used.
© 2012 Wiley Periodicals, Inc.
Figures

Similar articles
-
Comparative study of two techniques of mycophenolate mofetyl monitoring.Tunis Med. 2019 Aug-Sep;97(8-9):1010-1016. Tunis Med. 2019. PMID: 32173850
-
CEDIA mycophenolic acid assay compared with HPLC-UV in specimens from transplant recipients.Ther Drug Monit. 2006 Oct;28(5):632-6. doi: 10.1097/01.ftd.0000243963.53322.8d. Ther Drug Monit. 2006. PMID: 17038877
-
Mathematical equations to calculate true mycophenolic acid concentration in human plasma by using two immunoassays with different cross-reactivities with acyl glucuronide metabolite: comparison of calculated values with values obtained by using an HPLC-UV method.J Clin Lab Anal. 2013 Jul;27(4):290-3. doi: 10.1002/jcla.21599. J Clin Lab Anal. 2013. PMID: 23852786 Free PMC article.
-
Therapeutic Drug Monitoring of Mycophenolic Acid.Adv Clin Chem. 2016;76:165-84. doi: 10.1016/bs.acc.2016.04.001. Epub 2016 Jun 11. Adv Clin Chem. 2016. PMID: 27645819 Review.
-
Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients.Ther Drug Monit. 2000 Feb;22(1):20-6. doi: 10.1097/00007691-200002000-00004. Ther Drug Monit. 2000. PMID: 10688252 Review.
References
-
- Wu JC. Mycophenolate mofetil: Molecular mechanisms of action. Perspex Drug Discov Design 1994;2:185–204.
-
- Bullingham RE, Nicholls AJ, Kamm BR. Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 1998;34:429–455. - PubMed
-
- Korecka M, Nikolic D, van Breemen RB, Shaw LM. Inhibition of inosine monophosphate dehydrogenase by mycophenolic acid glucuronide is attributable to the presence of trace quantities of mycophenolic acid. Clin Chem 1999;45:1047–1050. - PubMed
-
- Kaplan B, Meier‐Kriesche HU, Friedman G, et al. The effect of renal insufficiency on mycophenolic acid protein binding. J Clin Pharmacol 1999;39:715–720. - PubMed
-
- Tredger JM, Brown NW, Adams J, et al. Monitoring mycophenolate in liver transplant recipients: Toward a therapeutic range. Liver Transpl 2004;10:492–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

