Axon tracts guide zebrafish facial branchiomotor neuron migration through the hindbrain
- PMID: 23325758
- PMCID: PMC3557781
- DOI: 10.1242/dev.087148
Axon tracts guide zebrafish facial branchiomotor neuron migration through the hindbrain
Abstract
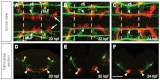
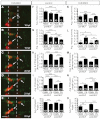
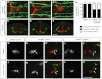
Appropriate localization of neurons within the brain is a crucial component of the establishment of neural circuitry. In the zebrafish hindbrain, the facial branchiomotor neurons (FBMNs) undergo a chain-like tangential migration from their birthplace in rhombomere (r) 4 to their final destination in r6/r7. Here, we report that ablation of either the cell body or the trailing axon of the leading FBMN, or 'pioneer' neuron, blocks the migration of follower FBMNs into r5. This demonstrates that the pioneer neuron and its axon are crucial to the early migration of FBMNs. Later migration from r5 to r6 is not dependent on pioneer neurons but on the medial longitudinal fasciculus (MLF), a bundle of axons lying ventral to the FBMNs. We find that MLF axons enter r5 only after the pioneer neuron has led several followers into this region; the MLF is then contacted by projections from the FBMNs. The interactions between FBMNs and the MLF are important for migration from r5 to r6, as blocking MLF axons from entering the hindbrain can stall FBMN migration in r5. Finally, we have found that the adhesion molecule Cdh2 (N-cadherin) is important for interactions between the MLF and FBMNs, as well as for interactions between the trailing axon of the pioneer neuron and follower FBMNs. Interestingly, migration of pioneer neurons is independent of both the MLF and Cdh2, suggesting pioneer migration relies on independent cues.
Figures





Similar articles
-
Rest represses maturation within migrating facial branchiomotor neurons.Dev Biol. 2015 May 15;401(2):220-35. doi: 10.1016/j.ydbio.2015.02.021. Epub 2015 Mar 11. Dev Biol. 2015. PMID: 25769695 Free PMC article.
-
Multiple mechanisms mediate motor neuron migration in the zebrafish hindbrain.Dev Neurobiol. 2010 Feb;70(2):87-99. doi: 10.1002/dneu.20761. Dev Neurobiol. 2010. PMID: 19937772 Free PMC article.
-
Cadherin-2 Is Required Cell Autonomously for Collective Migration of Facial Branchiomotor Neurons.PLoS One. 2016 Oct 7;11(10):e0164433. doi: 10.1371/journal.pone.0164433. eCollection 2016. PLoS One. 2016. PMID: 27716840 Free PMC article.
-
Facial motor neuron migration advances.Curr Opin Neurobiol. 2013 Dec;23(6):943-50. doi: 10.1016/j.conb.2013.09.001. Epub 2013 Sep 30. Curr Opin Neurobiol. 2013. PMID: 24090878 Free PMC article. Review.
-
Moving cell bodies: understanding the migratory mechanism of facial motor neurons.Arch Pharm Res. 2007 Oct;30(10):1273-82. doi: 10.1007/BF02980268. Arch Pharm Res. 2007. PMID: 18038906 Review.
Cited by
-
Potential Involvement of Draxin in the Axonal Projection of Cranial Nerves, Especially Cranial Nerve X, in the Chick Hindbrain.J Histochem Cytochem. 2016 Jul;64(7):412-24. doi: 10.1369/0022155416646538. Epub 2016 May 19. J Histochem Cytochem. 2016. PMID: 27199282 Free PMC article.
-
Myosin phosphatase Fine-tunes Zebrafish Motoneuron Position during Axonogenesis.PLoS Genet. 2016 Nov 17;12(11):e1006440. doi: 10.1371/journal.pgen.1006440. eCollection 2016 Nov. PLoS Genet. 2016. PMID: 27855159 Free PMC article.
-
Follow-the-leader cell migration requires biased cell-cell contact and local microenvironmental signals.Phys Biol. 2013 Jun;10(3):035003. doi: 10.1088/1478-3975/10/3/035003. Epub 2013 Jun 4. Phys Biol. 2013. PMID: 23735560 Free PMC article.
-
Development and migration of the zebrafish rhombencephalic octavolateral efferent neurons.J Comp Neurol. 2021 May 1;529(7):1293-1307. doi: 10.1002/cne.25021. Epub 2020 Sep 11. J Comp Neurol. 2021. PMID: 32869305 Free PMC article.
-
N-cadherin facilitates trigeminal sensory neuron outgrowth and target tissue innervation.Development. 2025 May 1;152(9):dev204369. doi: 10.1242/dev.204369. Epub 2025 May 1. Development. 2025. PMID: 40260574 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

