Glycosaminoglycan glycomics using mass spectrometry
- PMID: 23325770
- PMCID: PMC3617335
- DOI: 10.1074/mcp.R112.026294
Glycosaminoglycan glycomics using mass spectrometry
Abstract
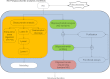
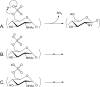
The fact that sulfated glycosaminoglycans (GAGs) are necessary for the functioning of all animal physiological systems drives the need to understand their biology. This understanding is limited, however, by the heterogeneous nature of GAG chains and their dynamic spatial and temporal expression patterns. GAGs have a regulated structure overlaid by heterogeneity but lack the detail necessary to build structure/function relationships. In order to provide this information, we need glycomics platforms that are sensitive, robust, high throughput, and information rich. This review summarizes progress on mass-spectrometry-based GAG glycomics methods. The areas covered include disaccharide analysis, oligosaccharide profiling, and tandem mass spectrometric sequencing.
Figures


References
-
- DeAngelis P. L. (2002) Evolution of glycosaminoglycans and their glycosyltransferases: implications for the extracellular matrices of animals and the capsules of pathogenic bacteria. Anat. Rec. 268, 317–326 - PubMed
-
- Bishop J. R., Schuksz M., Esko J. D. (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 - PubMed
-
- Bulow H. E., Hobert O. (2006) The molecular diversity of glycosaminoglycans shapes animal development. Annu. Rev. Cell Dev. Biol. 22, 375–407 - PubMed
-
- Chu C. L., Goerges A. L., Nugent M. A. (2005) Identification of common and specific growth factor binding sites in heparan sulfate proteoglycans. Biochemistry 44, 12203–12213 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

