Total and putative surface proteomics of malaria parasite salivary gland sporozoites
- PMID: 23325771
- PMCID: PMC3650326
- DOI: 10.1074/mcp.M112.024505
Total and putative surface proteomics of malaria parasite salivary gland sporozoites
Abstract
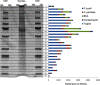
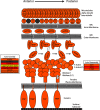
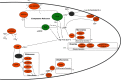
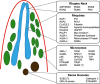
Malaria infections of mammals are initiated by the transmission of Plasmodium salivary gland sporozoites during an Anopheles mosquito vector bite. Sporozoites make their way through the skin and eventually to the liver, where they infect hepatocytes. Blocking this initial stage of infection is a promising malaria vaccine strategy. Therefore, comprehensively elucidating the protein composition of sporozoites will be invaluable in identifying novel targets for blocking infection. Previous efforts to identify the proteins expressed in Plasmodium mosquito stages were hampered by the technical difficulty of separating the parasite from its vector; without effective purifications, the large majority of proteins identified were of vector origin. Here we describe the proteomic profiling of highly purified salivary gland sporozoites from two Plasmodium species: human-infective Plasmodium falciparum and rodent-infective Plasmodium yoelii. The combination of improved sample purification and high mass accuracy mass spectrometry has facilitated the most complete proteome coverage to date for a pre-erythrocytic stage of the parasite. A total of 1991 P. falciparum sporozoite proteins and 1876 P. yoelii sporozoite proteins were identified, with >86% identified with high sequence coverage. The proteomic data were used to confirm the presence of components of three features critical for sporozoite infection of the mammalian host: the sporozoite motility and invasion apparatus (glideosome), sporozoite signaling pathways, and the contents of the apical secretory organelles. Furthermore, chemical labeling and identification of proteins on live sporozoites revealed previously uncharacterized complexity of the putative sporozoite surface-exposed proteome. Taken together, the data constitute the most comprehensive analysis to date of the protein expression of salivary gland sporozoites and reveal novel potential surface-exposed proteins that might be valuable targets for antibody blockage of infection.
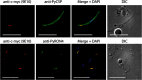
Figures
References
-
- Sinnis P., Zavala F. (2008) The skin stage of malaria infection: biology and relevance to the malaria vaccine effort. Future Microbiol. 3, 275–278 - PubMed
-
- Kappe S. H., Vaughan A. M., Boddey J. A., Cowman A. F. (2010) That was then but this is now: malaria research in the time of an eradication agenda. Science 328, 862–866 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

