An autonomous chromatin/DNA-PK mechanism for localized DNA damage signaling in mammalian cells
- PMID: 23325849
- PMCID: PMC3597672
- DOI: 10.1093/nar/gks1478
An autonomous chromatin/DNA-PK mechanism for localized DNA damage signaling in mammalian cells
Abstract
Rapid phosphorylation of histone variant H2AX proximal to DNA breaks is an initiating event and a hallmark of eukaryotic DNA damage responses. Three mammalian kinases are known to phosphorylate H2AX in response to DNA damage. However, the mechanism(s) for damage-localized phosphorylation remains incompletely understood. The DNA-dependent protein kinase (DNA-PK) is the most abundant H2AX-modifying kinases and uniquely activated by binding DNA termini. Here, we have developed a novel approach to examine enzyme activity and substrate properties by executing biochemical assays on intact cellular structures. We apply this approach to examine the mechanisms of localized protein modification in chromatin within fixed cells. DNA-PK retains substrate specificity and independently generates break-localized γH2AX foci in chromatin. In situ DNA-PK activity recapitulates localization and intensity of in vivo H2AX phosphorylation and requires no active cellular processes. Nuclease treatments or addition of exogenous DNA resulted in genome-wide H2AX phosphorylation, showing that DNA termini dictated the locality of H2AX phosphorylation in situ. DNA-PK also reconstituted focal phosphorylation of structural maintenance of chromatin protein 1, but not activating transcription factor 2. Allosteric regulation of DNA-PK by DNA termini protruding from chromatin constitutes an autonomous mechanism for break-localized protein phosphorylation that generates sub-nuclear foci. We discuss generalized implications of this mechanism in localizing mammalian DNA damage responses.
Figures
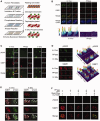
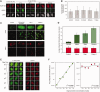
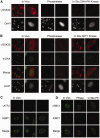
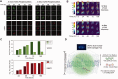
Similar articles
-
AIF-mediated caspase-independent necroptosis requires ATM and DNA-PK-induced histone H2AX Ser139 phosphorylation.Cell Death Dis. 2012 Sep 13;3(9):e390. doi: 10.1038/cddis.2012.120. Cell Death Dis. 2012. PMID: 22972376 Free PMC article.
-
DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells.DNA Repair (Amst). 2006 May 10;5(5):575-90. doi: 10.1016/j.dnarep.2006.01.011. Epub 2006 Mar 29. DNA Repair (Amst). 2006. PMID: 16567133
-
A kinome-targeted RNAi-based screen links FGF signaling to H2AX phosphorylation in response to radiation.Cell Mol Life Sci. 2015 Sep;72(18):3559-73. doi: 10.1007/s00018-015-1901-7. Epub 2015 Apr 18. Cell Mol Life Sci. 2015. PMID: 25894690 Free PMC article.
-
Assembly and function of DNA double-strand break repair foci in mammalian cells.DNA Repair (Amst). 2010 Dec 10;9(12):1219-28. doi: 10.1016/j.dnarep.2010.09.010. Epub 2010 Oct 28. DNA Repair (Amst). 2010. PMID: 21035408 Review.
-
Spatiotemporal characterization of ionizing radiation induced DNA damage foci and their relation to chromatin organization.Mutat Res. 2010 Apr-Jun;704(1-3):78-87. doi: 10.1016/j.mrrev.2009.12.006. Epub 2010 Jan 8. Mutat Res. 2010. PMID: 20060491 Free PMC article. Review.
Cited by
-
ATM/Wip1 activities at chromatin control Plk1 re-activation to determine G2 checkpoint duration.EMBO J. 2017 Jul 14;36(14):2161-2176. doi: 10.15252/embj.201696082. Epub 2017 Jun 12. EMBO J. 2017. PMID: 28607002 Free PMC article.
-
Induction and Processing of the Radiation-Induced Gamma-H2AX Signal and Its Link to the Underlying Pattern of DSB: A Combined Experimental and Modelling Study.PLoS One. 2015 Jun 11;10(6):e0129416. doi: 10.1371/journal.pone.0129416. eCollection 2015. PLoS One. 2015. PMID: 26067661 Free PMC article.
-
Activation-induced cytidine deaminase (AID) is necessary for the epithelial-mesenchymal transition in mammary epithelial cells.Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):E2977-86. doi: 10.1073/pnas.1301021110. Epub 2013 Jul 23. Proc Natl Acad Sci U S A. 2013. PMID: 23882083 Free PMC article.
-
Pericentric major satellite transcription is essential for meiotic chromosome stability and spindle pole organization.Open Biol. 2023 Nov;13(11):230133. doi: 10.1098/rsob.230133. Epub 2023 Nov 8. Open Biol. 2023. PMID: 37935356 Free PMC article.
-
DNA-PK and P38 MAPK: A Kinase Collusion in Alzheimer's Disease?Brain Disord Ther. 2017;6(2):232. doi: 10.4172/2168-975X.1000232. Epub 2017 May 1. Brain Disord Ther. 2017. PMID: 28706768 Free PMC article.
References
-
- Luger K, Rechsteiner TJ, Flaus AJ, Waye MM, Richmond TJ. Characterization of nucleosome core particles containing histone proteins made in bacteria. J. Mol. Biol. 1997;272:301–311. - PubMed
-
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–967. - PubMed
-
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273:5858–5868. - PubMed
-
- Bekker-Jensen S, Mailand N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair (Amst) 2010;9:1219–1228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

