Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma
- PMID: 23325902
- PMCID: PMC3589289
- DOI: 10.1212/WNL.0b013e3182815428
Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma
Abstract
Objective: To report long-term efficacy and safety data for everolimus for the treatment of subependymal giant cell astrocytoma (SEGA) in patients with tuberous sclerosis complex (TSC).
Methods: This was an open-label extension phase of a prospective, phase 1-2 trial (NCT00411619) in patients ≥3 years of age with SEGA associated with TSC. Patients received oral everolimus starting at 3 mg/m2 per day and subsequently titrated, subject to tolerability, to attain whole blood trough concentrations of 5-15 ng/mL. Change in SEGA volume, seizures, and safety assessments were the main outcome measures.
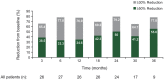
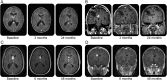
Results: Of 28 patients enrolled, 25 were still under treatment at the time of analysis. Median dose was 5.3 mg/m2/day and median treatment duration was 34.2 months (range 4.7-47.1). At all time points (18, 24, 30, and 36 months), primary SEGA volume was reduced by ≥30% from baseline (treatment response) in 65%-79% of patients. All patients reported ≥1 adverse event (AE), mostly grade 1/2 in severity, consistent with that previously reported, and none led to everolimus discontinuation. The most commonly reported drug-related AEs were upper respiratory infections (85.7%), stomatitis (85.7%), sinusitis (46.4%), and otitis media (35.7%). No drug-related grade 4 or 5 events occurred.
Conclusion: Everolimus therapy is safe and effective for longer term (median exposure 34.2 months) treatment of patients with TSC with SEGA.
Classification of evidence: This study provides Class III evidence that everolimus, titrated to trough serum levels of 5-15 ng/mL, was effective in reducing tumor size in patients with SEGA secondary to TSC for a median of 34 months.
Figures

References
-
- Baskin HJ., Jr The pathogenesis and imaging of the tuberous sclerosis complex. Pediatr Radiol 2008;38:936–952 - PubMed
-
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345–1356 - PubMed
-
- Goh S, Butler W, Thiele EA. Subependymal giant cell tumors in tuberous sclerosis complex. Neurology 2004;63:1457–1461 - PubMed
-
- Adriaensen ME, Schaefer-Prokop CM, Stijnen T, et al. Prevalence of subependymal giant cell tumors in patients with tuberous sclerosis and a review of the literature. Eur J Neurol 2009;16:691–696 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
