Kinetics of thermal degradation of betamethasone valerate and betamethasone dipropionate in different media
- PMID: 23325994
- PMCID: PMC3546330
- DOI: 10.4103/0250-474X.103845
Kinetics of thermal degradation of betamethasone valerate and betamethasone dipropionate in different media
Abstract
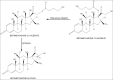
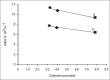
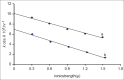
The effect of pH, media, phosphate concentration and ionic strength on the kinetics of thermal degradation of betamethasone valerate and betamethasone dipropionate has been investigated. A validated HPLC method has been used to determine the parent compounds and their major thermal degradation products identified in the reaction. Betamethasone-17-valerate gave rise to two major products, namely, betamethasone-21-valerate and betamethasone alcohol, and betamethasone dipropionate degraded into three major products, namely, betamethasone-17-propionate, betamethasone-21-propionate and betamethasone alcohol, in different media. Betamethasone valerate showed maximum stability at pH 4-5 while betamethasone dipropionate was maximally stable at pH 3.5-4.5. The degradation of betamethasone valerate and betamethasone dipropionate was found to follow first-order kinetics and the apparent first-order rate constants (k(obs)) for thermal degradation in different media range from 0.399-9.07×10(-3) h(-1) and 0.239-1.87×10(-3) h(-1), respectively. The values of the rate constants decrease with increasing solvent polarity, phosphate concentration and ionic strength. The second-order rate constants (k΄) for the phosphate ion inhibited reactions lie in the range of 3.02-1.30×10(-6) M(-1) s(-1).
Keywords: Betamethasone valerate; betamethasone dipropionate; kinetics; thermal degradation.
Figures




Similar articles
-
Photodegradation and stabilization of betamethasone-17 valerate in aqueous/organic solvents and topical formulations.AAPS PharmSciTech. 2013 Mar;14(1):177-82. doi: 10.1208/s12249-012-9902-4. Epub 2012 Dec 19. AAPS PharmSciTech. 2013. PMID: 23250710 Free PMC article.
-
High-performance liquid chromatographic assay of betamethasone 17-valerate and its degradation products.J Chromatogr. 1979 Sep 1;176(3):399-405. doi: 10.1016/s0021-9673(00)89458-3. J Chromatogr. 1979. PMID: 546923
-
Analysis of salicylic acid, arbutin and corticosteroids in skin whitening creams available in Pakistan using chromatographic techniques.Int J Cosmet Sci. 2016 Aug;38(4):421-8. doi: 10.1111/ics.12310. Epub 2016 Apr 5. Int J Cosmet Sci. 2016. PMID: 26855207
-
Fixed-dose combination therapy for psoriasis.Am J Clin Dermatol. 2004;5(2):71-7. doi: 10.2165/00128071-200405020-00001. Am J Clin Dermatol. 2004. PMID: 15109271 Review.
-
Spotlight on calcipotriene/betamethasone dipropionate in psoriasis vulgaris of the trunk, limbs, and scalp.Am J Clin Dermatol. 2011 Dec 1;12(6):421-4. doi: 10.2165/11207670-000000000-00000. Am J Clin Dermatol. 2011. PMID: 21967117 Review.
Cited by
-
Betamethasone Dipropionate Derivatization, Biotransformation, Molecular Docking, and ADME Analysis as Glucocorticoid Receptor.Biomed Res Int. 2022 Jul 6;2022:6865472. doi: 10.1155/2022/6865472. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2024 Jan 9;2024:9810723. doi: 10.1155/2024/9810723. PMID: 35865666 Free PMC article. Retracted.
-
Photodegradation and stabilization of betamethasone-17 valerate in aqueous/organic solvents and topical formulations.AAPS PharmSciTech. 2013 Mar;14(1):177-82. doi: 10.1208/s12249-012-9902-4. Epub 2012 Dec 19. AAPS PharmSciTech. 2013. PMID: 23250710 Free PMC article.
References
-
- Sweetman SC, editor. 36th ed. London: Pharmaceutical Press; 2009. Martindale The Complete Drug Reference.
-
- Council of Europe. 5th ed. France: Strasbourg, Cedex; 2004. European Pharmacopeia; pp. 1090–2. (1094-5).
-
- London: Her Majesty's Stationary Office; 2009. British Pharmacopoeia; pp. 251–2. (254-5).
-
- 32nd ed. Rockville, MD: United States Pharmacopeial Convention; 2009. United States Pharmacopeia; pp. 1660–1. (1665-6).
-
- Hamlin WE, Chulski T, Johnson RH, Wagner JG. A note on the photolytic degradation of antiinflammatory steroids. J Am Pharm Assoc. 1960;49:253–5. - PubMed
LinkOut - more resources
Full Text Sources
