Mechanisms of cholecystokinin-induced calcium mobilization in gastric antral interstitial cells of Cajal
- PMID: 23326123
- PMCID: PMC3544020
- DOI: 10.3748/wjg.v18.i48.7184
Mechanisms of cholecystokinin-induced calcium mobilization in gastric antral interstitial cells of Cajal
Abstract
Aim: To investigate the effect of sulfated cholecystokinin-8 (CCK-8S) on calcium mobilization in cultured murine gastric antral interstitial cells of Cajal (ICC) and its possible mechanisms.
Methods: ICC were isolated from the gastric antrum of mice and cultured. Immunofluorescence staining with a monoclonal antibody for c-Kit was used to identify ICC. The responsiveness of ICC to CCK-8S was measured using Fluo-3/AM based digital microfluorimetric measurement of intracellular Ca(2+) concentration ([Ca(2+)]i). A confocal laser scanning microscope was used to monitor [Ca(2+)]i changes. The selective CCK(1) receptor antagonist lorglumide, the intracellular Ca(2+)-ATPase inhibitor thapsigargin, the type III inositol 1,4,5-triphosphate (InsP(3)) receptor blocker xestospongin C and the L-type voltage-operated Ca(2+) channel inhibitor nifedipine were used to examine the mechanisms of [Ca(2+)]i elevation caused by CCK-8S. Immunoprecipitation and Western blotting were used to determine the regulatory effect of PKC on phosphorylation of type III InsP(3) receptor (InsP(3)R3) in ICC. Protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (PMA) and inhibitor chelerythrine were used to assess the role of PKC in the CCK-8S-evoked [Ca(2+)]i increment of ICC.
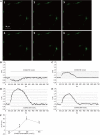
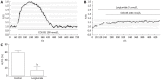
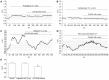
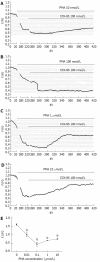
Results: ICC were successfully isolated from the gastric antrum of mice and cultured. Cultured ICC were identified by immunofluorescence staining. When given 80 nmol/L or more than 80 nmol/L CCK-8S, the [Ca(2+)]i in ICC increased and 100 nmol/L CCK-8S significantly increased the mean [Ca(2+)]i by 59.30% ± 4.85% (P < 0.01). Pretreatment of ICC with 5 μmol/L lorglumide inhibited 100 nmol/L CCK-8S-induced [Ca(2+)]i increment from 59.30% ± 4.85% to 14.97% ± 9.05% (P < 0.01), suggesting a CCK(1)R-mediated event. Emptying of intracellular calcium stores by thapsigargin (5 μmol/L) prevented CCK-8S (100 nmol/L) from inducing a [Ca(2+)]i increase. Moreover, pretreatment with xestospongin C (1 μmol/L) could also abolish the CCK-8S-induced effect, indicating that Ca(2+) release from InsP(3)R-operated stores appeared to be a major mechanism responsible for CCK-8S-induced calcium mobilization in ICC. On the other hand, by removing extracellular calcium or blocking the L-type voltage-operated calcium channel with nifedipine, a smaller but significant rise in the [Ca(2+)]i could be still elicited by CCK-8S. These data suggest that the [Ca(2+)]i release is not stimulated or activated by the influx of extracellular Ca(2+) in ICC, but the influx of extracellular Ca(2+) can facilitate the [Ca(2+)]i increase evoked by CCK-8S. CCK-8S increased the phosphorylation of InsP(3)R3, which could be prevented by chelerythrine. Pretreatment with lorglumide (5 μmol/L) could significantly reduce the CCK-8S intensified phosphorylation of InsP(3)R3. In the positive control group, treatment of cells with PMA also resulted in an enhanced phosphorylation of InsP(3)R3. Pretreatment with various concentrations of PMA (10 nmol/L-10 μmol/L) apparently inhibited the effect of CCK-8S and the effect of 100 nmol/L PMA was most obvious. Likewise, the effect of CCK-8S was augmented by the pretreatment with chelerythrine (10 nmol/L-10 μmol/L) and 100 nmol/L chelerythrine exhibited the maximum effect.
Conclusion: CCK-8S increases [Ca(2+)]i in ICC via the CCK(1) receptor. This effect depends on the release of InsP(3)R-operated Ca(2+) stores, which is negatively regulated by PKC-mediated phosphorylation of InsP(3)R3.
Keywords: Calcium mobilization; Cholecystokinin octapeptide; Interstitial cells of Cajal; Protein kinase C.
Figures



Similar articles
-
[The role of protein kinase C-mediated phosphorylation of type III inositol 1, 4, 5-triphosphate receptor in cholecystokinin octapeptide induced calcium mobilization in gastric antral smooth muscle cells].Zhonghua Yi Xue Za Zhi. 2007 Mar 13;87(10):664-9. Zhonghua Yi Xue Za Zhi. 2007. PMID: 17553302 Chinese.
-
Signal transduction pathways mediating CCK-8S-induced gastric antral smooth muscle contraction.Digestion. 2006;73(4):249-58. doi: 10.1159/000095628. Epub 2006 Sep 5. Digestion. 2006. PMID: 16954694
-
Effects of cholecystokinin-8 induced gastric dysmotility on bile regurgitation during stress and molecular mechanisms.Regul Pept. 2006 Sep 11;136(1-3):64-71. doi: 10.1016/j.regpep.2006.04.016. Epub 2006 Jun 30. Regul Pept. 2006. PMID: 16814406
-
Role of calcium in activation of hyperpolarization-activated cyclic nucleotide-gated channels caused by cholecystokinin octapeptide in interstitial cells of cajal.Digestion. 2012;85(4):266-75. doi: 10.1159/000337077. Epub 2012 Apr 24. Digestion. 2012. PMID: 22538231
-
[The effect and mechanism of cholecystokinin octapeptide induced-gastric dysmotility on bile regurgitation during stress].Zhonghua Nei Ke Za Zhi. 2006 Oct;45(10):827-30. Zhonghua Nei Ke Za Zhi. 2006. PMID: 17217748 Chinese.
Cited by
-
Roles of interleukin-9 in the growth and cholecystokinin-induced intracellular calcium signaling of cultured interstitial cells of Cajal.PLoS One. 2014 Apr 22;9(4):e95898. doi: 10.1371/journal.pone.0095898. eCollection 2014. PLoS One. 2014. PMID: 24755995 Free PMC article.
-
Improvement of functional dyspepsia with Suaeda salsa (L.) Pall via regulating brain-gut peptide and gut microbiota structure.Eur J Nutr. 2024 Aug;63(5):1929-1944. doi: 10.1007/s00394-024-03401-2. Epub 2024 May 4. Eur J Nutr. 2024. PMID: 38703229
-
Interstitial cells of Cajal: update on basic and clinical science.Curr Gastroenterol Rep. 2014 Jan;16(1):363. doi: 10.1007/s11894-013-0363-z. Curr Gastroenterol Rep. 2014. PMID: 24408748 Review.
References
-
- Boddy G, Bong A, Cho W, Daniel EE. ICC pacing mechanisms in intact mouse intestine differ from those in cultured or dissected intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G653–G662. - PubMed
-
- Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

