Asymmetric PTEN distribution regulated by spatial heterogeneity in membrane-binding state transitions
- PMID: 23326224
- PMCID: PMC3542079
- DOI: 10.1371/journal.pcbi.1002862
Asymmetric PTEN distribution regulated by spatial heterogeneity in membrane-binding state transitions
Abstract
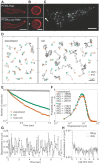
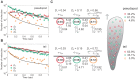
The molecular mechanisms that underlie asymmetric PTEN distribution at the posterior of polarized motile cells and regulate anterior pseudopod formation were addressed by novel single-molecule tracking analysis. Heterogeneity in the lateral mobility of PTEN on a membrane indicated the existence of three membrane-binding states with different diffusion coefficients and membrane-binding lifetimes. The stochastic state transition kinetics of PTEN among these three states were suggested to be regulated spatially along the cell polarity such that only the stable binding state is selectively suppressed at the anterior membrane to cause local PTEN depletion. By incorporating experimentally observed kinetic parameters into a simple mathematical model, the asymmetric PTEN distribution can be explained quantitatively to illustrate the regulatory mechanisms for cellular asymmetry based on an essential causal link between individual stochastic reactions and stable localizations of the ensemble.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Mutual inhibition between PTEN and PIP3 generates bistability for polarity in motile cells.Nat Commun. 2018 Oct 26;9(1):4481. doi: 10.1038/s41467-018-06856-0. Nat Commun. 2018. PMID: 30367048 Free PMC article.
-
Single-molecule imaging of PI(4,5)P2 and PTEN in vitro reveals a positive feedback mechanism for PTEN membrane binding.Commun Biol. 2020 Feb 28;3(1):92. doi: 10.1038/s42003-020-0818-3. Commun Biol. 2020. PMID: 32111929 Free PMC article.
-
Different modes of state transitions determine pattern in the Phosphatidylinositide-Actin system.BMC Cell Biol. 2011 Oct 7;12:42. doi: 10.1186/1471-2121-12-42. BMC Cell Biol. 2011. PMID: 21982379 Free PMC article.
-
Regulation of PTEN function as a PIP3 gatekeeper through membrane interaction.Cell Cycle. 2006 Jul;5(14):1523-7. doi: 10.4161/cc.5.14.3005. Epub 2006 Jul 17. Cell Cycle. 2006. PMID: 16861931 Review.
-
Temporal and spatial regulation of chemotaxis.Dev Cell. 2002 Oct;3(4):469-78. doi: 10.1016/s1534-5807(02)00292-7. Dev Cell. 2002. PMID: 12408799 Review.
Cited by
-
Estimation of diffusion constants from single molecular measurement without explicit tracking.BMC Syst Biol. 2018 Apr 11;12(Suppl 1):15. doi: 10.1186/s12918-018-0526-5. BMC Syst Biol. 2018. PMID: 29671388 Free PMC article.
-
PTEN hopping on the cell membrane is regulated via a positively-charged C2 domain.PLoS Comput Biol. 2014 Sep 11;10(9):e1003817. doi: 10.1371/journal.pcbi.1003817. eCollection 2014 Sep. PLoS Comput Biol. 2014. PMID: 25211206 Free PMC article.
-
Physics of compartmentalization: How phase separation and signaling shape membrane and organelle identity.Comput Struct Biotechnol J. 2021 May 24;19:3225-3233. doi: 10.1016/j.csbj.2021.05.029. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34141141 Free PMC article. Review.
-
Single particle tracking-based reaction progress kinetic analysis reveals a series of molecular mechanisms of cetuximab-induced EGFR processes in a single living cell.Chem Sci. 2017 Jul 1;8(7):4823-4832. doi: 10.1039/c7sc01159h. Epub 2017 Apr 24. Chem Sci. 2017. PMID: 28959404 Free PMC article.
-
Modeling self-organized spatio-temporal patterns of PIP₃ and PTEN during spontaneous cell polarization.Phys Biol. 2014 Aug;11(4):046002. doi: 10.1088/1478-3975/11/4/046002. Epub 2014 Jul 15. Phys Biol. 2014. PMID: 25024302 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

