Neem leaf glycoprotein activates CD8(+) T cells to promote therapeutic anti-tumor immunity inhibiting the growth of mouse sarcoma
- PMID: 23326300
- PMCID: PMC3543399
- DOI: 10.1371/journal.pone.0047434
Neem leaf glycoprotein activates CD8(+) T cells to promote therapeutic anti-tumor immunity inhibiting the growth of mouse sarcoma
Abstract
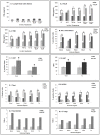
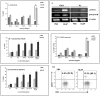
In spite of sufficient data on Neem Leaf Glycoprotein (NLGP) as a prophylactic vaccine, little knowledge currently exists to support the use of NLGP as a therapeutic vaccine. Treatment of mice bearing established sarcomas with NLGP (25 µg/mice/week subcutaneously for 4 weeks) resulted in tumor regression or dormancy (Tumor free/Regressor, 13/24 (NLGP), 4/24 (PBS)). Evaluation of CD8(+) T cell status in blood, spleen, TDLN, VDLN and tumor revealed increase in cellular number. Elevated expression of CD69, CD44 and Ki67 on CD8(+) T cells revealed their state of activation and proliferation by NLGP. Depletion of CD8(+) T cells in mice at the time of NLGP treatment resulted in partial termination of tumor regression. An expansion of CXCR3(+) and CCR5(+) T cells was observed in the TDLN and tumor, along with their corresponding ligands. NLGP treatment enhances type 1 polarized T-bet expressing T cells with downregulation of GATA3. Treg cell population was almost unchanged. However, T∶Treg ratios significantly increased with NLGP. Enhanced secretion/expression of IFNγ was noted after NLGP therapy. In vitro culture of T cells with IL-2 and sarcoma antigen resulted in significant enhancement in cytotoxic efficacy. Consistently higher expression of CD107a was also observed in CD8(+) T cells from tumors. Reinoculation of sarcoma cells in tumor regressed NLGP-treated mice maintained tumor free status in majority. This is correlated with the increment of CD44(hi)CD62L(hi) central memory T cells. Collectively, these findings support a paradigm in which NLGP dynamically orchestrates the activation, expansion, and recruitment of CD8(+) T cells into established tumors to operate significant tumor cell lysis.
Conflict of interest statement
Figures




Similar articles
-
Normalization of tumor microenvironment by neem leaf glycoprotein potentiates effector T cell functions and therapeutically intervenes in the growth of mouse sarcoma.PLoS One. 2013 Jun 13;8(6):e66501. doi: 10.1371/journal.pone.0066501. Print 2013. PLoS One. 2013. PMID: 23785504 Free PMC article.
-
Neem leaf glycoprotein promotes dual generation of central and effector memory CD8(+) T cells against sarcoma antigen vaccine to induce protective anti-tumor immunity.Mol Immunol. 2016 Mar;71:42-53. doi: 10.1016/j.molimm.2016.01.007. Epub 2016 Feb 3. Mol Immunol. 2016. PMID: 26851529
-
Immunotherapeutic targeting of established sarcoma in Swiss mice by tumor-derived antigen-pulsed NLGP matured dendritic cells is CD8+ T-cell dependent.Immunotherapy. 2014;6(7):821-31. doi: 10.2217/imt.14.53. Immunotherapy. 2014. PMID: 25290415
-
Neem Leaf Glycoprotein in immunoregulation of cancer.Hum Immunol. 2022 Nov;83(11):768-777. doi: 10.1016/j.humimm.2022.08.012. Epub 2022 Aug 31. Hum Immunol. 2022. PMID: 36055899 Review.
-
Targeting CD8+ T cells with natural products for tumor therapy: Revealing insights into the mechanisms.Phytomedicine. 2024 Jul;129:155608. doi: 10.1016/j.phymed.2024.155608. Epub 2024 Apr 8. Phytomedicine. 2024. PMID: 38642413 Review.
Cited by
-
Normalization of tumor microenvironment by neem leaf glycoprotein potentiates effector T cell functions and therapeutically intervenes in the growth of mouse sarcoma.PLoS One. 2013 Jun 13;8(6):e66501. doi: 10.1371/journal.pone.0066501. Print 2013. PLoS One. 2013. PMID: 23785504 Free PMC article.
-
Antimicrobial Efficacy of Three Different Mouthwashes on Periodontal Pathogens-An In vitro Study.J Pharm Bioallied Sci. 2023 Jul;15(Suppl 1):S459-S462. doi: 10.4103/jpbs.jpbs_612_22. Epub 2023 Jul 5. J Pharm Bioallied Sci. 2023. PMID: 37654410 Free PMC article.
-
Integrative vision in cancer research, prevention and therapy.J Ayurveda Integr Med. 2024 Jan-Feb;15(1):100856. doi: 10.1016/j.jaim.2023.100856. Epub 2024 Jan 3. J Ayurveda Integr Med. 2024. PMID: 38176303 Free PMC article.
-
Neem leaf glycoprotein prophylaxis transduces immune dependent stop signal for tumor angiogenic switch within tumor microenvironment.PLoS One. 2014 Nov 12;9(11):e110040. doi: 10.1371/journal.pone.0110040. eCollection 2014. PLoS One. 2014. PMID: 25391149 Free PMC article.
-
Exploring the therapeutic potential of Neem (Azadirachta Indica) for the treatment of prostate cancer: a literature review.Ann Transl Med. 2022 Jul;10(13):754. doi: 10.21037/atm-22-94. Ann Transl Med. 2022. PMID: 35957716 Free PMC article. Review.
References
-
- Angevin E, Kremer F, Gaudin C, Hercend T, Triebel F (1997) Analysis of T-cell immune response in renal cell carcinoma: polarization to type 1-like differentiation pattern, clonal T-cell expansion and tumor-specific cytotoxicity. Int J Cancer 72: 431–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

