Use of a single hybrid imaging agent for integration of target validation with in vivo and ex vivo imaging of mouse tumor lesions resembling human DCIS
- PMID: 23326303
- PMCID: PMC3543428
- DOI: 10.1371/journal.pone.0048324
Use of a single hybrid imaging agent for integration of target validation with in vivo and ex vivo imaging of mouse tumor lesions resembling human DCIS
Abstract
Screening of biomarker expression levels in tumor biopsy samples not only provides an assessment of prognostic and predictive factors, but may also be used for selection of biomarker-specific imaging strategies. To assess the feasibility of using a biopsy specimen for a personalized selection of an imaging agent, the chemokine receptor 4 (CXCR4) was used as a reference biomarker.
Methods: A hybrid CXCR4 targeting peptide (MSAP-Ac-TZ14011) containing a fluorescent dye and a chelate for radioactive labeling was used to directly compare initial flow cytometry-based target validation in fresh tumor tissue to in vivo single photon emission computed tomography (SPECT) imaging and in vivo and ex vivo fluorescence imaging.
Results: Flow cytometric analysis of mouse tumor derived cell suspensions enabled discrimination between 4T1 control tumor lesions (with low levels of CXCR4 expression) and CXCR4 positive early, intermediate and late stage MIN-O lesions based on their CXCR4 expression levels; CXCR4(basal), CXCR4(+) and CXCR4(++) cell populations could be accurately discriminated. Mean fluorescent intensity ratios between expression in MIN-O and 4T1 tissue found with flow cytometry were comparable to ratios obtained with in vivo SPECT/CT and fluorescence imaging, ex vivo fluorescence evaluation and standard immunohistochemistry.
Conclusion: The hybrid nature of a targeting imaging agent like MSAP-Ac-TZ14011 enables integration of target selection, in vivo imaging and ex vivo validation using a single agent. The use of biopsy tissue for biomarker screening can readily be expanded to other targeting hybrid imaging agents and can possibly help increase the clinical applicability of tumor-specific imaging approaches.
Conflict of interest statement
Figures

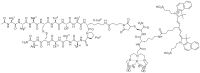




Similar articles
-
Hybrid Imaging Labels: Providing the Link Between Mass Spectrometry-Based Molecular Pathology and Theranostics.Theranostics. 2017 Jan 12;7(3):624-633. doi: 10.7150/thno.17484. eCollection 2017. Theranostics. 2017. PMID: 28255355 Free PMC article.
-
Non-invasive longitudinal imaging of tumor progression using an (111)indium labeled CXCR4 peptide antagonist.Am J Nucl Med Mol Imaging. 2012;2(1):99-109. Epub 2011 Dec 15. Am J Nucl Med Mol Imaging. 2012. PMID: 23133805 Free PMC article.
-
Fluorescent CXCR4 targeting peptide as alternative for antibody staining in Ewing sarcoma.BMC Cancer. 2017 May 26;17(1):383. doi: 10.1186/s12885-017-3352-z. BMC Cancer. 2017. PMID: 28549419 Free PMC article.
-
111In-Labeled Ac-TZ14011 peptide (a chemokine receptor 4 antagonist) conjugated to CyAL5.5 (a fluorescent dye) through a multifunctional single-attachment point (MSAP) reagent.2012 Nov 29 [updated 2012 Dec 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Nov 29 [updated 2012 Dec 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23285497 Free Books & Documents. Review.
-
Molecular imaging of chemokine receptor CXCR4.Theranostics. 2013;3(1):76-84. doi: 10.7150/thno.4835. Epub 2013 Jan 15. Theranostics. 2013. PMID: 23382787 Free PMC article. Review.
Cited by
-
Emerging Fluorescent Molecular Tracers to Guide Intra-Operative Surgical Decision-Making.Front Pharmacol. 2019 May 14;10:510. doi: 10.3389/fphar.2019.00510. eCollection 2019. Front Pharmacol. 2019. PMID: 31139085 Free PMC article. Review.
-
Translation of c-Met Targeted Image-Guided Surgery Solutions in Oral Cavity Cancer-Initial Proof of Concept Data.Cancers (Basel). 2021 May 28;13(11):2674. doi: 10.3390/cancers13112674. Cancers (Basel). 2021. PMID: 34071623 Free PMC article.
-
Hybrid surgical guidance based on the integration of radionuclear and optical technologies.Br J Radiol. 2016 Jun;89(1062):20150797. doi: 10.1259/bjr.20150797. Epub 2016 Mar 4. Br J Radiol. 2016. PMID: 26943463 Free PMC article. Review.
-
Hybrid Imaging Labels: Providing the Link Between Mass Spectrometry-Based Molecular Pathology and Theranostics.Theranostics. 2017 Jan 12;7(3):624-633. doi: 10.7150/thno.17484. eCollection 2017. Theranostics. 2017. PMID: 28255355 Free PMC article.
-
Biomarkers in preclinical cancer imaging.Eur J Nucl Med Mol Imaging. 2015 Apr;42(4):579-96. doi: 10.1007/s00259-014-2980-7. Epub 2015 Feb 12. Eur J Nucl Med Mol Imaging. 2015. PMID: 25673052 Free PMC article. Review.
References
-
- Allred DC (2010) Issues and updates: evaluating estrogen receptor-alpha, progesterone receptor, and HER2 in breast cancer. Modern Pathology 23 Suppl 2: S52–9. - PubMed
-
- Pritchard KI, Shepherd KI, O'Malley FP, Andruli IL, Tu D, et al. (2006) National Cancer Institute of Canada Clinical Trials Group, HER2 and responsiveness of breast cancer to adjuvant chemotherapy. New Eng J Med 354 (20) 2103–11. - PubMed
-
- Penault-Llorca F, Cayre A, Bouchet Mishellany F, Amat S, Feillel V, et al. (2003) Induction chemotherapy for breast carcinoma: predictive markers and relation with outcome. Int J Oncol 22 (6) 1319–25. - PubMed
-
- (IBSG), I.B.C.S.G (2002) Endocrine responsiveness and tailoring adjuvant therapy for postmenopauzal lymph node-negative breast cancer: A randomized trail. J Natl Cancer Inst 94 (14) 1054–1065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

