Apolipoprotein(a) inhibits in vitro tube formation in endothelial cells: identification of roles for Kringle V and the plasminogen activation system
- PMID: 23326327
- PMCID: PMC3543409
- DOI: 10.1371/journal.pone.0052287
Apolipoprotein(a) inhibits in vitro tube formation in endothelial cells: identification of roles for Kringle V and the plasminogen activation system
Abstract
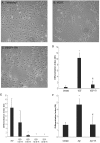
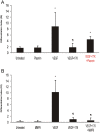
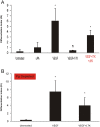
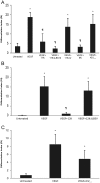
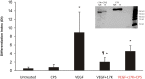
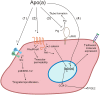
Elevated plasma concentrations of lipoprotein(a) are associated with increased risk for atherothrombotic diseases. Apolipoprotein(a), the unique glycoprotein component of lipoprotein(a), is characterized by the presence of multiple kringle domains, and shares a high degree of sequence homology with the serine protease zymogen plasminogen. It has been shown that angiostatin, a proteolytic fragment of plasminogen containing kringles 1-4, can effectively inhibit angiogenesis. Moreover, proteolytic fragments of plasminogen containing kringle 5 are even more potent inhibitors of angiogenesis than angiostatin. Despite its strong similarity with plasminogen, the role of apolipoprotein(a) in angiogenesis remains controversial, with both pro- and anti-angiogenic effects reported. In the current study, we evaluated the ability of apolipoprotein(a) to inhibit VEGF- and angiopoietin-induced tube formation in human umbilical cord endothelial cells. A 17 kringle-containing form of recombinant apo(a) (17K), corresponding to a well-characterized, physiologically-relevant form of the molecule, effectively inhibited tube formation induced by either VEGF or angiopoietin-1. Using additional recombinant apolipoprotein(a) (r-apo(a)) variants, we demonstrated that this effect was dependent on the presence of an intact lysine-binding site in kringle V domain of apo(a), but not on the presence of the functional lysine-binding site in apo(a) kringle IV type 10; sequences within in the amino-terminal half of the molecule were also not required for the inhibitory effects of apo(a). We also showed that the apo(a)-mediated inhibition tube formation could be reversed, in part by the addition of plasmin or urokinase plasminogen activator, or by removal of plasminogen from the system. Further, we demonstrated that apo(a) treated with glycosidases to remove sialic acid was significantly less effective in inhibiting tube formation. This is the first report of a functional role for the glycosylation of apo(a) although the mechanisms underlying this observation remain to be determined in the context of angiogenesis.
Conflict of interest statement
Figures
References
-
- Tsimikas S, Hall JL (2012) Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease. J Am Coll Cardiol 60: 716–721. - PubMed
-
- Koschinsky ML, Côté GP, Gabel B, van der Hoek YY (1993) Identification of the cysteine residue in apolipoprotein(a) that mediates extracellular coupling with apolipoprotein B-100. J Biol Chem 268: 19819–19825. - PubMed
-
- McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, et al. (1987) cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature 330: 132–137. - PubMed
-
- Koschinsky ML, Marcovina SM (2009) Lipoprotein(a). In Clinical Lipidology: A Companion to Braunwald's Heart Disease. (ed. C.M. Ballantyne), Saunders (Elsevier), Philadelphia. pp. 130–143.
-
- van der Hoek YY, Wittekoek ME, Beisiegel U, Kastelein JJ, Koschinsky ML (1993) The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum Mol Genet 2: 361–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

