Activated ERM protein plays a critical role in drug resistance of MOLT4 cells induced by CCL25
- PMID: 23326330
- PMCID: PMC3541277
- DOI: 10.1371/journal.pone.0052384
Activated ERM protein plays a critical role in drug resistance of MOLT4 cells induced by CCL25
Abstract
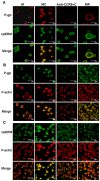
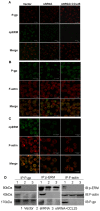
We have previously demonstrated that the CCR9/CCL25 signaling pathway plays an important role in drug resistance in human acute T-lymphocytic leukemia (T-ALL) by inducing activation of ERM protein with polarized distribution in T-ALL cell line MOLT4. However, the mechanism of action of the activated ERM protein in the drug resistance of MOLT4 cells induced by CCL25 remains uncharacterized. Here we investigated the mechanism of CCR9/CCL25-initiated drug resistance in CCR9-high-expressing T-ALL cells. Our results showed that 1) the function of P-gp was increased after treatment with CCL25; 2) P-gp colocalized and co-immunoprecipitated with p-ERM and F-actin in CCL25 treated cells; and 3) ERM-shRNA conferred drug sensitivity coincident with release of ERM interactions with P-gp and F-actin after treatment with CCL25. These data suggest it is pivotal that P-gp associate with the F-actin cytoskeleton through p-ERM in CCR9/CCL25 induced multidrug resistance of T-ALL cells. Strategies aimed at inhibiting P-gp-F-actin cytoskeleton association may be helpful in increasing the efficiency of therapies in T-ALL.
Conflict of interest statement
Figures






References
-
- Pui CH, Behm FG, Crist WM (1993) Clinical and biologic relevance of immunologic marker studies in childhood acute lymphoblastic leukemia. Blood 82: 343–362. - PubMed
-
- Willemse MJ, Seriu T, Hettinger K, d'Aniello E, Hop WC, et al. (2002) Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood 99 (12) 4386–4393. - PubMed
-
- Gottesman MM, Ling V (2006) The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett 580: 998–1009. - PubMed
-
- Sharon FJ (2008) ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 9 (1) 105–127. - PubMed
-
- Meschini S, Calcabrini A, Monti E, Del Bufalo D, Stringaro A, et al. (2000) Intracellular P-glycoprotein expression is associated with the intrinsic multidrug resistance phenotype in human colon adenocarcinoma cells. Int J Cancer 87 (5) 615–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

