24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy
- PMID: 23326337
- PMCID: PMC3543414
- DOI: 10.1371/journal.pone.0052512
24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy
Erratum in
- PLoS One. 2013;8(11). doi:10.1371/annotation/cbe611fe-cda9-4d98-9574-0ac18e109daa
Abstract
Objectives: The aim of the study was i) to assess the spectrum of changes over 24 months in ambulant boys affected by Duchenne muscular dystrophy, ii) to establish the difference between the first and the second year results and iii) to identify possible early markers of loss of ambulation.
Methods: One hundred and thirteen patients (age range 4.1-17, mean 8.2) fulfilled the inclusion criteria, 67 of the 113 were on daily and 40 on intermittent steroids, while 6 were not on steroids. All were assessed using the 6 Minute Walk Test (6MWT), the North Star Ambulatory Assessment (NSAA) and timed test.
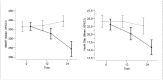
Results: On the 6MWT there was an average overall decline of -22.7 (SD 81.0) in the first year and of -64.7 (SD 123.1) in the second year. On the NSAA the average overall decline was of -1.86 (SD 4.21) in the first year and of -2.98 (SD 5.19) in the second year. Fourteen children lost ambulation, one in the first year and the other 13 in the second year of the study. A distance of at least 330 meters on the 6MWT, or a NSAA score of 18 at baseline reduced significantly the risk of losing ambulation within 2 years.
Conclusions: These results can be of help at the time of using inclusion criteria for a study in ambulant patients in order to minimize the risk of patients who may lose ambulation within the time of the trial.
Conflict of interest statement
Figures
References
-
- McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, et al. (2010) The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve 41: 500–510. - PubMed
-
- Mazzone E, Martinelli D, Berardinelli A, Messina S, D’Amico A, et al. (2010) North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord 20: 712–716. - PubMed
-
- Mazzone E, Vasco G, Sormani MP, Torrente Y, Berardinelli A, et al. (2011) Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology 77: 250–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources