Sickle erythrocytes target cytotoxics to hypoxic tumor microvessels and potentiate a tumoricidal response
- PMID: 23326340
- PMCID: PMC3541382
- DOI: 10.1371/journal.pone.0052543
Sickle erythrocytes target cytotoxics to hypoxic tumor microvessels and potentiate a tumoricidal response
Abstract
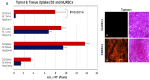
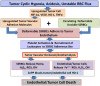
Resistance of hypoxic solid tumor niches to chemotherapy and radiotherapy remains a major scientific challenge that calls for conceptually new approaches. Here we exploit a hitherto unrecognized ability of sickled erythrocytes (SSRBCs) but not normal RBCs (NLRBCs) to selectively target hypoxic tumor vascular microenviroment and induce diffuse vaso-occlusion. Within minutes after injection SSRBCs, but not NLRBCs, home and adhere to hypoxic 4T1 tumor vasculature with hemoglobin saturation levels at or below 10% that are distributed over 70% of the tumor space. The bound SSRBCs thereupon form microaggregates that obstruct/occlude up to 88% of tumor microvessels. Importantly, SSRBCs, but not normal RBCs, combined with exogenous prooxidant zinc protoporphyrin (ZnPP) induce a potent tumoricidal response via a mutual potentiating mechanism. In a clonogenic tumor cell survival assay, SSRBC surrogate hemin, along with H(2)O(2) and ZnPP demonstrate a similar mutual potentiation and tumoricidal effect. In contrast to existing treatments directed only to the hypoxic tumor cell, the present approach targets the hypoxic tumor vascular environment and induces injury to both tumor microvessels and tumor cells using intrinsic SSRBC-derived oxidants and locally generated ROS. Thus, the SSRBC appears to be a potent new tool for treatment of hypoxic solid tumors, which are notable for their resistance to existing cancer treatments.
Conflict of interest statement
Figures






Similar articles
-
Sickle cells produce functional immune modulators and cytotoxics.Am J Hematol. 2017 Oct;92(10):981-988. doi: 10.1002/ajh.24836. Epub 2017 Aug 17. Am J Hematol. 2017. PMID: 28646491 Free PMC article.
-
Exogenous sickle erythrocytes combined with vascular disruption trigger disseminated tumor vaso-occlusion and lung tumor regression.JCI Insight. 2019 Apr 4;4(7):e125535. doi: 10.1172/jci.insight.125535. eCollection 2019 Apr 4. JCI Insight. 2019. PMID: 30944254 Free PMC article.
-
Drug-loaded sickle cells programmed ex vivo for delayed hemolysis target hypoxic tumor microvessels and augment tumor drug delivery.J Control Release. 2013 Oct 28;171(2):184-92. doi: 10.1016/j.jconrel.2013.07.008. Epub 2013 Jul 18. J Control Release. 2013. PMID: 23871960 Free PMC article.
-
Heme degradation and vascular injury.Antioxid Redox Signal. 2010 Feb;12(2):233-48. doi: 10.1089/ars.2009.2822. Antioxid Redox Signal. 2010. PMID: 19697995 Free PMC article. Review.
-
Sickle cell vasoocclusion: heterotypic, multicellular aggregations driven by leukocyte adhesion.Microcirculation. 2004 Mar;11(2):167-77. Microcirculation. 2004. PMID: 15280090 Review.
Cited by
-
A Bioreductive Prodrug of Cucurbitacin B Significantly Inhibits Tumor Growth in the 4T1 Xenograft Mice Model.ACS Med Chem Lett. 2019 Sep 10;10(10):1400-1406. doi: 10.1021/acsmedchemlett.9b00161. eCollection 2019 Oct 10. ACS Med Chem Lett. 2019. PMID: 31620225 Free PMC article.
-
Limitations of the dorsal skinfold window chamber model in evaluating anti-angiogenic therapy during early phase of angiogenesis.Vasc Cell. 2014 Aug 4;6:17. doi: 10.1186/2045-824X-6-17. eCollection 2014. Vasc Cell. 2014. PMID: 25101168 Free PMC article.
-
Automated synthesis of [68Ga]oxine, improved preparation of 68Ga-labeled erythrocytes for blood-pool imaging, and preclinical evaluation in rodents.Medchemcomm. 2018 Feb 1;9(3):454-459. doi: 10.1039/c7md00607a. eCollection 2018 Mar 1. Medchemcomm. 2018. PMID: 30108935 Free PMC article.
-
Sickle cells produce functional immune modulators and cytotoxics.Am J Hematol. 2017 Oct;92(10):981-988. doi: 10.1002/ajh.24836. Epub 2017 Aug 17. Am J Hematol. 2017. PMID: 28646491 Free PMC article.
-
Distearoyl anchor-painted erythrocytes with prolonged ligand retention and circulation properties in vivo.Adv Healthc Mater. 2014 Jan;3(1):142-8. doi: 10.1002/adhm.201300084. Epub 2013 Jun 25. Adv Healthc Mater. 2014. PMID: 23798381 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

