Pathway analysis reveals common pro-survival mechanisms of metyrapone and carbenoxolone after traumatic brain injury
- PMID: 23326402
- PMCID: PMC3541279
- DOI: 10.1371/journal.pone.0053230
Pathway analysis reveals common pro-survival mechanisms of metyrapone and carbenoxolone after traumatic brain injury
Abstract
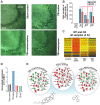
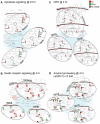
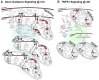
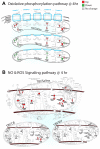
Developing new pharmacotherapies for traumatic brain injury (TBI) requires elucidation of the neuroprotective mechanisms of many structurally and functionally diverse compounds. To test our hypothesis that diverse neuroprotective drugs similarly affect common gene targets after TBI, we compared the effects of two drugs, metyrapone (MT) and carbenoxolone (CB), which, though used clinically for noncognitive conditions, improved learning and memory in rats and humans. Although structurally different, both MT and CB inhibit a common molecular target, 11β hydroxysteroid dehydrogenase type 1, which converts inactive cortisone to cortisol, thereby effectively reducing glucocorticoid levels. We examined injury-induced signaling pathways to determine how the effects of these two compounds correlate with pro-survival effects in surviving neurons of the injured rat hippocampus. We found that treatment of TBI rats with MT or CB acutely induced in hippocampal neurons transcriptional profiles that were remarkably similar (i.e., a coordinated attenuation of gene expression across multiple injury-induced cell signaling networks). We also found, to a lesser extent, a coordinated increase in cell survival signals. Analysis of injury-induced gene expression altered by MT and CB provided additional insight into the protective effects of each. Both drugs attenuated expression of genes in the apoptosis, death receptor and stress signaling pathways, as well as multiple genes in the oxidative phosphorylation pathway such as subunits of NADH dehydrogenase (Complex1), cytochrome c oxidase (Complex IV) and ATP synthase (Complex V). This suggests an overall inhibition of mitochondrial function. Complex 1 is the primary source of reactive oxygen species in the mitochondrial oxidative phosphorylation pathway, thus linking the protective effects of these drugs to a reduction in oxidative stress. The net effect of the drug-induced transcriptional changes observed here indicates that suppressing expression of potentially harmful genes, and also, surprisingly, reduced expression of pro-survival genes may be a hallmark of neuroprotective therapeutic effects.
Conflict of interest statement
Figures


Comment in
-
Translation experiments in traumatic brain injury … is it time to renew pharmacologic therapy?World Neurosurg. 2013 Nov;80(5):447-8. doi: 10.1016/j.wneu.2013.09.009. Epub 2013 Sep 12. World Neurosurg. 2013. PMID: 24035988 No abstract available.
References
-
- Schouten JW (2007) Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr Opin Crit Care 13: 134–142. - PubMed
-
- Arciniegas D, Adler L, Topkoff J, Cawthra E, Filley CM, et al. (1999) Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj 13: 1–13. - PubMed
-
- Zhang Z, Larner SF, Kobeissy F, Hayes RL, Wang KK (2010) Systems biology and theranostic approach to drug discovery and development to treat traumatic brain injury. Methods Mol Biol 662: 317–329. - PubMed
-
- Kasarskis A, Yang X, Schadt E (2011) Integrative genomics strategies to elucidate the complexity of drug response. Pharmacogenomics 12: 1695–1715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

