Metabolic side-effects of the novel second-generation antipsychotic drugs asenapine and iloperidone: a comparison with olanzapine
- PMID: 23326434
- PMCID: PMC3541274
- DOI: 10.1371/journal.pone.0053459
Metabolic side-effects of the novel second-generation antipsychotic drugs asenapine and iloperidone: a comparison with olanzapine
Abstract
Background: The second generation antipsychotic (SGA) drugs are widely used in psychiatry due to their clinical efficacy and low incidence of neurological side-effects. However, many drugs in this class cause deleterious metabolic side-effects. Animal models accurately predict metabolic side-effects for SGAs with known clinical metabolic liability. We therefore used preclinical models to evaluate the metabolic side-effects of glucose intolerance and insulin resistance with the novel SGAs asenapine and iloperidone for the first time. Olanzapine was used as a comparator.
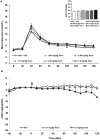
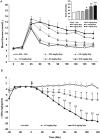
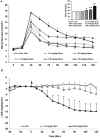
Methods: Adults female rats were treated with asenapine (0.01, 0.05, 0.1, 0.5, 1.0 mg/kg), iloperidone (0.03, 0.5, 1.0, 5.0, 10.0 mg/kg) or olanzapine (0.1, 0.5, 1.5, 5.0, 10.0 mg/kg) and subjected to the glucose tolerance test (GTT). Separate groups of rats were treated with asenapine (0.1 and 1.0 mg/kg), iloperidone (1.0 and 10 mg/kg) or olanzapine (1.5 and 15 mg/kg) and tested for insulin resistance with the hyperinsulinemic-euglycemic clamp (HIEC).
Results: Asenapine showed no metabolic effects at any dose in either test. Iloperidone caused large and significant glucose intolerance with the three highest doses in the GTT, and insulin resistance with both doses in the HIEC. Olanzapine caused significant glucose intolerance with the three highest doses in the GTT, and insulin resistance with the higher dose in the HIEC.
Conclusions: In preclinical models, asenapine shows negligible metabolic liability. By contrast, iloperidone exhibits substantial metabolic liability, comparable to olanzapine. These results emphasize the need for appropriate metabolic testing in patients treated with novel SGAs where current clinical data do not exist.
Conflict of interest statement
Figures
References
-
- Procyshyn RM, Honer WG, Wu TK, Ko RW, McIsaac SA, et al. (2010) Persistent antipsychotic polypharmacy and excessive dosing in the community psychiatric treatment setting: a review of medication profiles in 435 Canadian outpatients. J Clin Psychiatry 71: 566–573. - PubMed
-
- Newcomer JW (2007) Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry 68 Suppl 48–13. - PubMed
-
- Henderson DC (2008) Managing weight gain and metabolic issues in patients treated with atypical antipsychotics. J Clin Psychiatry 69: e04. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

