Antitumor activity of antimicrobial peptides containing CisoDGRC in CD13 negative breast cancer cells
- PMID: 23326440
- PMCID: PMC3543424
- DOI: 10.1371/journal.pone.0053491
Antitumor activity of antimicrobial peptides containing CisoDGRC in CD13 negative breast cancer cells
Abstract
Background: isoAsp-Gly-Arg (isoDGR) is a derivative of the Asn-Gly-Arg (NGR) motif, which is used as a targeted delivery tool to aminopeptidase N (CD13) positive cells. Recent studies have shown that cyclic isoDGR (CisoDGRC) has a more efficient affinity with α(v)β(3), a type of integrin that overexpresses in tumor cells. Antimicrobial peptides (AMPs) are an efficient antitumor peptide that specifically kills tumor cells. In the present study, we designed antimicrobial peptides containing the CisoDGRC motif (CDAK) and assessed its antitumor activity for CD13(-)/α(v)β(3) (+) breast cancer cells (MCF-7 and MDA-MB-231) in vitro and in vivo.
In vitro: We assessed the cytotoxicity of CDAK for MCF-7 and MDA-MB-231 breast cancer cells, the human umbilical vein endothelial cell (HUVEC), and human foreskin fibroblasts (HFF). We performed an apoptosis assay using Annexin-V/PI, DNA ladder, mitochondrial membrane potential, and Caspase-3 and Bcl-2. The effect on cell cycles and affinity with cell were tested using flow cytometry and fluorescent microscopy and the effect on invasion was analyzed using an invasion assay. CDAK was injected intravenously into tumor-bearing athymic nude mice in vivo experiment.
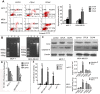
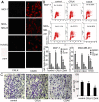
Results: CDAK showed cytotoxic activity in MCF-7 and MDA-MB-231 cells, whereas HUVEC and HFF were less sensitive to the peptides. CDAK induced apoptosis, reduced mitochondrial membrane potential, promoted Caspase-3, and inhibited Bcl-2 expression in the two breast cancer cell lines. In addition, CDAK inhibited proliferation of cancer cell through S phase arrest, and own selective affinity with MCF-7 and MDA-MB-231cells, inhibited the invasion of MDA-MB-231 cells. In vivo, CDAK significant inhibited the progression of the tumor and the generation of neovascularization.
Conclusion: Antimicrobial peptides containing the CisoDGRC (CDAK) motif could efficiently exhibit the antitumor activity for CD13(-)/α(v)β(3) (+) breast cancer cells.
Conflict of interest statement
Figures


References
-
- Gregorc V, De Braud FG, De Pas TM, Scalamogna R, Citterio G, et al. (2011) Phase I study of NGR-hTNF, a selective vascular targeting agent, in combination with cisplatin in refractory solid tumors. Clin Cancer Res 17: 1964–1972. - PubMed
-
- Rizzardi GP, Bordignon C (2009) NGR and isoDGR are separate moieties binding to different receptors. Blood 113: 5366; author reply 5367. - PubMed
-
- Corti A, Curnis F (2011) Isoaspartate-dependent molecular switches for integrin-ligand recognition. J Cell Sci 124: 515–522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

