Tumor-derived autophagosomes (DRibbles) induce B cell activation in a TLR2-MyD88 dependent manner
- PMID: 23326458
- PMCID: PMC3541185
- DOI: 10.1371/journal.pone.0053564
Tumor-derived autophagosomes (DRibbles) induce B cell activation in a TLR2-MyD88 dependent manner
Abstract
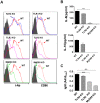
Previously, we have documented that isolated autophagosomes from tumor cells could efficiently cross-prime tumor-reactive naïve T cells and mediate tumor regression in preclinical mouse models. However, the effect of tumor-derived autophagosomes, here we refer as to DRibbles, on B cells has not been studied so far. At present study, we found that DRibbles generated from a murine hepatoma cell line Hep1-6, induced B-cell activation after intravenous injection into mice. B-cell populations were significantly expanded and the production of Hep1-6 tumor-specific antibodies was successfully induced. Moreover, in vitro studies showed that DRibbles could induce more efficient B-cell proliferation and activation, antibody production, and cytokine secretion than whole tumor cell lysates. Notably, we found that B-cell activation required proteins but not DNA in the DRibbles. We further showed that B cells could capture DRibbles and present antigens in the DRibbles to directly induce T cell activation. Furthermore, we found that B-cell activation, antibody production, cytokine secretion and antigen cross-presentation were TLR2-MyD88 pathway dependent. Taken together, the present studies demonstrated that tumor-derived autophagosomes (DRibbles) efficiently induced B cells activation, antibody production, cytokine secretion and antigen cross-presentation mainly depending on their protein component via TLR2/MyD88 dependent manner.
Conflict of interest statement
Figures
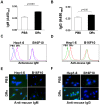
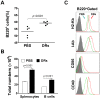
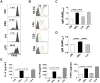
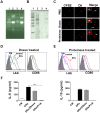


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

