bis-Dehydroxy-Curcumin triggers mitochondrial-associated cell death in human colon cancer cells through ER-stress induced autophagy
- PMID: 23326480
- PMCID: PMC3543386
- DOI: 10.1371/journal.pone.0053664
bis-Dehydroxy-Curcumin triggers mitochondrial-associated cell death in human colon cancer cells through ER-stress induced autophagy
Abstract
Background: The activation of autophagy has been extensively described as a pro-survival strategy, which helps to keep cells alive following deprivation of nutrients/growth factors and other stressful cellular conditions. In addition to cytoprotective effects, autophagy can accompany cell death. Autophagic vacuoles can be observed before or during cell death, but the role of autophagy in the death process is still controversial. A complex interplay between autophagy and apoptosis has come to light, taking into account that numerous genes, such as p53 and Bcl-2 family members, are shared between these two pathways.
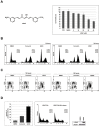
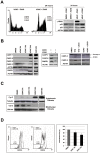
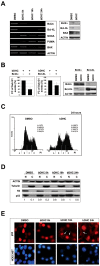
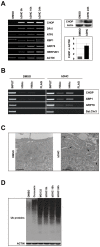
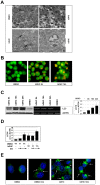
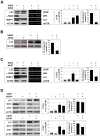
Methodology/principal findings: In this study we showed a potent and irreversible cytotoxic activity of the stable Curcumin derivative bis-DeHydroxyCurcumin (bDHC) on human colon cancer cells, but not on human normal cells. Autophagy is elicited by bDHC before cell death as demonstrated by increased autophagosome formation -measured by electron microscopy, fluorescent LC3 puncta and LC3 lipidation- and autophagic flux -measured by interfering LC3-II turnover. The accumulation of poly-ubiquitinated proteins and ER-stress occurred upstream of autophagy induction and resulted in cell death. Cell cycle and Western blot analyses highlighted the activation of a mitochondrial-dependent apoptosis, which involves caspase 7, 8, 9 and Cytochrome C release. Using pharmacological inhibitions and RNAi experiments, we showed that ER-stress induced autophagy has a major role in triggering bDHC-cell death.
Conclusion/significance: Our findings describe the mechanism through which bDHC promotes tumor selective inhibition of proliferation, providing unequivocal evidence of the role of autophagy in contrasting the proliferation of colon cancer cells.
Conflict of interest statement
Figures


References
-
- Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9: 231–241. - PubMed
-
- Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2: 277–288. - PubMed
-
- Cuervo AM (2004) Autophagy: many paths to the same end. Mol Cell Biochem 263: 55–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

