Genetic and functional dissection of ARMS2 in age-related macular degeneration and polypoidal choroidal vasculopathy
- PMID: 23326481
- PMCID: PMC3541181
- DOI: 10.1371/journal.pone.0053665
Genetic and functional dissection of ARMS2 in age-related macular degeneration and polypoidal choroidal vasculopathy
Abstract
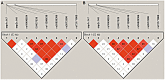
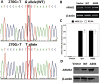
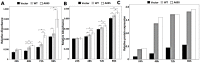
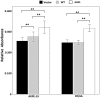
Age-related maculopathy susceptibility 2(ARMS2) was suggested to be associated with neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV) in multiple genetic studies in Caucasians and Japanese. To date, no biological properties have been attributed to the putative protein in nAMD and PCV. The complete genes of ARMS2 and HTRA1 including all exons and the promoter region were assessed using direct sequencing technology in 284 unrelated mainland northern Chinese individuals: 96 nAMD patients, 92 PCV patients and 96 controls. Significant associations with both nAMD and PCV were observed in 2 polymorphisms of ARMS2 and HTRA1 rs11200638, with different genotypic distributions between nAMD and PCV (p<0.001). After adjusting for rs11200638, ARMS2 rs10490924 remained significantly associated with nAMD and PCV (p<0.001). Then we overexpressed wild-type ARMS2 and ARMS2 A69S mutation (rs10490924) in RF/6A cells and RPE cells as in vitro study model. Cell proliferation, attachment, migration and tube formation were analyzed for the first time. Compare with wild-type ARMS2, A69S mutation resulted in a significant increase in proliferation and attachment but inhibited cell migration. Moreover, neither wild-type ARMS2 nor A69S mutation affected tube formation of RF/6A cells. There is a strong and consistent association of the ARMS2/HTRA1 locus with both nAMD and PCV, suggesting the two disorders share, at least partially, similar molecular mechanisms. Neither wild-type ARMS2 nor A69S mutation had direct association with neovascularisation in the pathogenesis of AMD.
Conflict of interest statement
Figures


References
-
- Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, et al.. (2004) Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004/04/14 ed. 477–485. - PubMed
-
- Fine SL, Berger JW, Maguire MG, Ho AC (2000) Age-related macular degeneration. N Engl J Med 342: 483–492. - PubMed
-
- Jager RD, Mieler WF, Miller JW (2008) Age-related macular degeneration. N Engl J Med 358: 2606–2617. - PubMed
-
- Liu NP (2009) [Characteristics of age-related macular degeneration in Chinese population]. Zhonghua Yan Ke Za Zhi 45: 393–395. - PubMed
-
- Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya’ale D, et al. (2004) 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol 11: 67–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

