Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer
- PMID: 23326489
- PMCID: PMC3543364
- DOI: 10.1371/journal.pone.0053701
Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer
Abstract
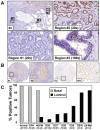
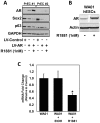
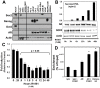
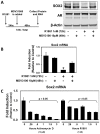
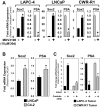
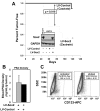
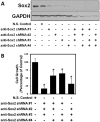
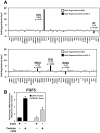
Despite advances in detection and therapy, castration-resistant prostate cancer continues to be a major clinical problem. The aberrant activity of stem cell pathways, and their regulation by the Androgen Receptor (AR), has the potential to provide insight into novel mechanisms and pathways to prevent and treat advanced, castrate-resistant prostate cancers. To this end, we investigated the role of the embryonic stem cell regulator Sox2 [SRY (sex determining region Y)-box 2] in normal and malignant prostate epithelial cells. In the normal prostate, Sox2 is expressed in a portion of basal epithelial cells. Prostate tumors were either Sox2-positive or Sox2-negative, with the percentage of Sox2-positive tumors increasing with Gleason Score and metastases. In the castration-resistant prostate cancer cell line CWR-R1, endogenous expression of Sox2 was repressed by AR signaling, and AR chromatin-IP shows that AR binds the enhancer element within the Sox2 promoter. Likewise, in normal prostate epithelial cells and human embryonic stem cells, increased AR signaling also decreases Sox2 expression. Resistance to the anti-androgen MDV3100 results in a marked increase in Sox2 expression within three prostate cancer cell lines, and in the castration-sensitive LAPC-4 prostate cancer cell line ectopic expression of Sox2 was sufficient to promote castration-resistant tumor formation. Loss of Sox2 expression in the castration-resistant CWR-R1 prostate cancer cell line inhibited cell growth. Up-regulation of Sox2 was not associated with increased CD133 expression but was associated with increased FGF5 (Fibroblast Growth Factor 5) expression. These data propose a model of elevated Sox2 expression due to loss of AR-mediated repression during castration, and consequent castration-resistance via mechanisms not involving induction of canonical embryonic stem cell pathways.
Conflict of interest statement
Figures

References
-
- Huggins C, Stevens RE, Hodges CV (1941) Studies on Prostate Cancer: II. The Effects of Castration on Advanced Carcinoma of the Prostate Gland. Archives of Surgery 43: 209–223.
-
- Isaacs JT, Isaacs WB (2004) Androgen receptor outwits prostate cancer drugs. Nat Med 10: 26–27. - PubMed
-
- Singh P, Uzgare A, Litvinov I, Denmeade SR, Isaacs JT (2006) Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr Relat Cancer 13: 653–666. - PubMed
-
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. - PubMed
-
- Rodriguez-Pinilla SM, Sarrio D, Moreno-Bueno G, Rodriguez-Gil Y, Martinez MA, et al. (2007) Sox2: a possible driver of the basal-like phenotype in sporadic breast cancer. Mod Pathol 20: 474–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

