Downregulation of the mitochondrial phosphatase PTPMT1 is sufficient to promote cancer cell death
- PMID: 23326511
- PMCID: PMC3542197
- DOI: 10.1371/journal.pone.0053803
Downregulation of the mitochondrial phosphatase PTPMT1 is sufficient to promote cancer cell death
Abstract
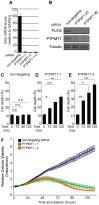
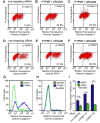
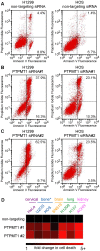
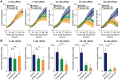
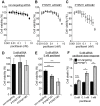
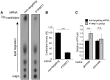
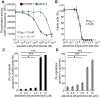
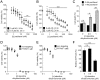
Protein Tyrosine Phosphatase localized to the Mitochondrion 1 (PTPMT1) is a dual specificity phosphatase exclusively localized to the mitochondria, and has recently been shown to be a critical component in the cardiolipin biosynthetic pathway. The downregulation of PTPMT1 in pancreatic beta cells has been shown to increase cellular ATP levels and insulin production, however, the generalized role of PTPMT1 in cancer cells has not been characterized. Here we report that downregulation of PTPMT1 activity is sufficient to induce apoptosis of cancer cells. Additionally, the silencing of PTPMT1 decreases cardiolipin levels in cancer cells, while selectively increasing ATP levels in glycolytic media. Additionally, sublethal downregulation of PTPMT1 synergizes with low doses of paclitaxel to promote cancer cell death. Our data suggest that inhibition of PTPMT1 causes a metabolic crisis in cancer cells that induces cell death, and may be a mechanism by which cancer cells can be sensitized to currently available therapies.
Conflict of interest statement
Figures
Similar articles
-
Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis.Cell Metab. 2011 Jun 8;13(6):690-700. doi: 10.1016/j.cmet.2011.04.007. Cell Metab. 2011. PMID: 21641550 Free PMC article.
-
The pseudophosphatase MK-STYX physically and genetically interacts with the mitochondrial phosphatase PTPMT1.PLoS One. 2014 Apr 7;9(4):e93896. doi: 10.1371/journal.pone.0093896. eCollection 2014. PLoS One. 2014. PMID: 24709986 Free PMC article.
-
Structural and functional analysis of PTPMT1, a phosphatase required for cardiolipin synthesis.Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11860-5. doi: 10.1073/pnas.1109290108. Epub 2011 Jul 5. Proc Natl Acad Sci U S A. 2011. PMID: 21730175 Free PMC article.
-
PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting.Adv Biol Regul. 2014 Sep;56:66-80. doi: 10.1016/j.jbior.2014.07.002. Epub 2014 Jul 19. Adv Biol Regul. 2014. PMID: 25088603 Review.
-
The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications.Curr Cancer Drug Targets. 2008 May;8(3):187-98. doi: 10.2174/156800908784293659. Curr Cancer Drug Targets. 2008. PMID: 18473732 Review.
Cited by
-
Drosophila PTPMT1 Has a Function in Tracheal Air Filling.iScience. 2020 Jul 24;23(7):101285. doi: 10.1016/j.isci.2020.101285. Epub 2020 Jun 20. iScience. 2020. PMID: 32629421 Free PMC article.
-
Splicing factor SRSF1 promotes breast cancer progression via oncogenic splice switching of PTPMT1.J Exp Clin Cancer Res. 2021 May 15;40(1):171. doi: 10.1186/s13046-021-01978-8. J Exp Clin Cancer Res. 2021. PMID: 33992102 Free PMC article.
-
Insertional hypermutation in mineral oil-induced plasmacytomas.Eur J Immunol. 2014 Sep;44(9):2785-801. doi: 10.1002/eji.201344322. Eur J Immunol. 2014. PMID: 24975032 Free PMC article.
-
ZFX controls propagation and prevents differentiation of acute T-lymphoblastic and myeloid leukemia.Cell Rep. 2014 Feb 13;6(3):528-40. doi: 10.1016/j.celrep.2014.01.007. Epub 2014 Jan 30. Cell Rep. 2014. PMID: 24485662 Free PMC article.
-
Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: its implication in cancer therapeutics.Cell Mol Life Sci. 2019 May;76(9):1641-1652. doi: 10.1007/s00018-018-2990-x. Epub 2018 Dec 11. Cell Mol Life Sci. 2019. PMID: 30539200 Free PMC article. Review.
References
-
- Soubannier V, McBride HM (2009) Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta 1793: 154–170. - PubMed
-
- Taylor EB, Rutter J (2011) Mitochondrial quality control by the ubiquitin-proteasome system. Biochem Soc Trans 39: 1509–1513. - PubMed
-
- Willers IM, Cuezva JM (2011) Post-transcriptional regulation of the mitochondrial H(+)-ATP synthase: a key regulator of the metabolic phenotype in cancer. Biochim Biophys Acta 1807: 543–551. - PubMed
-
- Pagliarini DJ, Dixon JE (2006) Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci 31: 26–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

