Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling
- PMID: 23326514
- PMCID: PMC3543305
- DOI: 10.1371/journal.pone.0053810
Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling
Abstract
The majority of ovarian primordial follicles must be preserved in a quiescent state to allow for the regular production of gametes over the female reproductive lifespan. However, the molecular mechanism that maintains the long quiescence of primordial follicles is poorly understood. Under certain pathological conditions, the entire pool of primordial follicles matures simultaneously leading to an accelerated loss of primordial follicles and to premature ovarian failure (POF). We have previously shown that loss of Pten (phosphatase and tensin homolog deleted on chromosome ten) in mouse oocytes leads to premature activation of the entire pool of primordial follicles, subsequent follicular depletion in early adulthood, and the onset of POF. Lack of PTEN leads to increased phosphatidylinositol 3-kinase (PI3K)-Akt and mammalian target of rapamycin complex 1 (mTORC1) signaling in the oocytes. To study the functional and pathological roles of elevated mTORC1 signaling in the oocytes, we treated the Pten-mutant mice with the specific mTORC1 inhibitor rapamycin. When administered to Pten-deficient mice prior to the activation of the primordial follicles, rapamycin effectively prevented global follicular activation and preserved the ovarian reserve. These results provide a rationale for exploring the possible use of rapamycin as a drug for the preservation of the primordial follicle pool, and the possible prevention of POF.
Conflict of interest statement
Figures

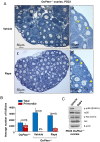
References
-
- Adhikari D, Liu K (2009) Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev 30: 438–464. - PubMed
-
- Reddy P, Zheng W, Liu K (2010) Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab 21: 96–103. - PubMed
-
- Broekmans FJ, Knauff EAH, te Velde ER, Macklon NS, Fauser BC (2007) Female reproductive ageing: current knowledge and future trends. Trends in Endocrinology & Metabolism 18: 58–65. - PubMed
-
- Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, et al. (2008) A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod 23: 699–708. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

