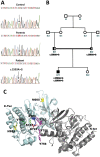
A mutation causes MuSK reduced sensitivity to agrin and congenital myasthenia
- PMID: 23326516
- PMCID: PMC3541344
- DOI: 10.1371/journal.pone.0053826
A mutation causes MuSK reduced sensitivity to agrin and congenital myasthenia
Erratum in
- PLoS One. 2013;8(9). doi: 10.1371/annotation/3ff2b918-c83c-4c6f-a2e2-4d91294ec92f
Abstract
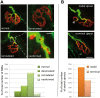
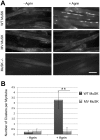
Congenital myasthenic syndromes (CMSs) are a heterogeneous group of genetic disorders affecting neuromuscular transmission. The agrin/muscle-specific kinase (MuSK) pathway is critical for proper development and maintenance of the neuromuscular junction (NMJ). We report here an Iranian patient in whom CMS was diagnosed since he presented with congenital and fluctuating bilateral symmetric ptosis, upward gaze palsy and slowly progressive muscle weakness leading to loss of ambulation. Genetic analysis of the patient revealed a homozygous missense mutation c.2503A>G in the coding sequence of MUSK leading to the p.Met835Val substitution. The mutation was inherited from the two parents who were heterozygous according to the notion of consanguinity. Immunocytochemical and electron microscopy studies of biopsied deltoid muscle showed dramatic changes in pre- and post-synaptic elements of the NMJs. These changes induced a process of denervation/reinnervation in native NMJs and the formation, by an adaptive mechanism, of newly formed and ectopic NMJs. Aberrant axonal outgrowth, decreased nerve terminal ramification and nodal axonal sprouting were also noted. In vivo electroporation of the mutated MuSK in a mouse model showed disorganized NMJs and aberrant axonal growth reproducing a phenotype similar to that observed in the patient's biopsy specimen. In vitro experiments showed that the mutation alters agrin-dependent acetylcholine receptor aggregation, causes a constitutive activation of MuSK and a decrease in its agrin- and Dok-7-dependent phosphorylation.
Conflict of interest statement
Figures







References
-
- Brockhausen J, Cole RN, Gervásio OL, Ngo ST, Noakes PG, et al. (2008) Neural agrin increases postsynaptic ACh receptor packing by elevating rapsyn protein at the mouse neuromuscular synapse. Dev Neurobiol 68: 1153–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

