Asymptomatic cattle naturally infected with Mycobacterium bovis present exacerbated tissue pathology and bacterial dissemination
- PMID: 23326525
- PMCID: PMC3541226
- DOI: 10.1371/journal.pone.0053884
Asymptomatic cattle naturally infected with Mycobacterium bovis present exacerbated tissue pathology and bacterial dissemination
Abstract
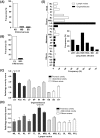
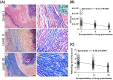
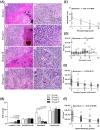
Rational discovery of novel immunodiagnostic and vaccine candidate antigens to control bovine tuberculosis (bTB) requires knowledge of disease immunopathogenesis. However, there remains a paucity of information on the Mycobacterium bovis-host immune interactions during the natural infection. Analysis of 247 naturally PPD+ M. bovis-infected cattle revealed that 92% (n = 228) of these animals were found to display no clinical signs, but presented severe as well as disseminated bTB-lesions at post-mortem examination. Moreover, dissemination of bTB-lesions positively correlated with both pathology severity score (Spearman r = 0.48; p<0.0001) and viable tissue bacterial loads (Spearman r = 0.58; p = 0.0001). Additionally, granuloma encapsulation negatively correlated with M. bovis growth as well as pathology severity, suggesting that encapsulation is an effective mechanism to control bacterial proliferation during natural infection. Moreover, multinucleated giant cell numbers were found to negatively correlate with bacterial counts (Spearman r = 0.25; p = 0.03) in lung granulomas. In contrast, neutrophil numbers in the granuloma were associated with increased M. bovis proliferation (Spearman r = 0.27; p = 0.021). Together, our findings suggest that encapsulation and multinucleated giant cells control M. bovis viability, whereas neutrophils may serve as a cellular biomarker of bacterial proliferation during natural infection. These data integrate host granuloma responses with mycobacterial dissemination and could provide useful immunopathological-based biomarkers of disease severity in natural infection with M. bovis, an important cattle pathogen.
Conflict of interest statement
Figures


References
-
- World Health Organization (2008) Global tuberculosis control - surveillance, planning, financing: WHO report 2008. WHO/HTM/TB/2008 393: 1–304.
-
- Thoen C, LoBue P, de Kantor I (2006) The importance of Mycobacterium bovis as a zoonosis. Vet Microbiol 112: 339–3345. - PubMed
-
- de Kantor IN, Ambroggi M, Poggi S, Morcillo N, Telles MADS, et al. (2008) Human Mycobacterium bovis infection in ten Latin American countries. Tuberculosis 88: 358–365. - PubMed
-
- Berg S, Firdessa R, Habtamu M, Gadisa E, Mengistu A, et al. (2009) The Burden of Mycobacterial Disease in Ethiopian Cattle: Implications for Public Health. PLoS One 4: e5068 doi:10.1371/journal.pone.0005068 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

