The expression of Pax6 variants is subject to posttranscriptional regulation in the developing mouse eyelid
- PMID: 23326536
- PMCID: PMC3542254
- DOI: 10.1371/journal.pone.0053919
The expression of Pax6 variants is subject to posttranscriptional regulation in the developing mouse eyelid
Abstract
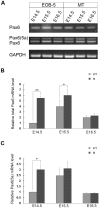
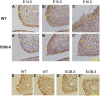
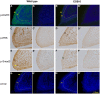
Pax6 is a pivotal transcription factor that plays a role during early eye morphogenesis, but its expression and function in eyelid development remain unknown. In this study, the expression patterns of Pax6 mRNA and protein were examined in the developing mouse eyelid at embryonic days 14.5, 15.5, and 16.5. The function of Pax6 in eyelid development was determined by comparing it to that in the eyes-open-at-birth mutant mouse. In the normally developing eyelid, Pax6 and Pax6(5a) mRNA levels were low at E14.5, increased at E15.5, and then declined at E16.5, accompanied by a change in the Pax6/Pax6(5a) ratio. Pax6 protein was mainly located in the mesenchyme and conjunctiva. It was expressed at low levels in the epidermis at E14.5, severely reduced at E15.5, but re-expressed in the keratinocyte cells of the periderm at E16.5. In contrast, Pax6 and the Pax6/Pax6(5a) ratio were considerably higher with strong nuclear expression in the mutant at E15.5. Next, we examined the relationship of Pax6 to epidermal cell proliferation, migration, and the associated signalling pathways. The Pax6 protein in the developing eyelid was negatively correlated with epidermal cell proliferation but not migration, and it is in contrast to the activation of the EGFR-ERK pathway. Our in vivo data suggest that Pax6 expression and the Pax6/Pax6(5a) ratio are at relatively low levels in the eyelid, and acting as a transcription factor, Pax6 is required for the initiation of eyelid formation and for differential development of the keratinised cells in the closed eyelid. The Pax6 protein is likely to be controlled by the EGFR-ERK pathways. An abnormal increase in Pax6 expression and the Pax6/Pax6(5a) ratio due to alteration of the pathway activity could suppress epidermal cell proliferation leading to the eyes-open-at-birth defect. This study offers insight into the function of the Pax6 protein in eyelid development.
Conflict of interest statement
Figures


Similar articles
-
Role of Fabp7, a downstream gene of Pax6, in the maintenance of neuroepithelial cells during early embryonic development of the rat cortex.J Neurosci. 2005 Oct 19;25(42):9752-61. doi: 10.1523/JNEUROSCI.2512-05.2005. J Neurosci. 2005. PMID: 16237179 Free PMC article.
-
Overexpression of Pax6 results in microphthalmia, retinal dysplasia and defective retinal ganglion cell axon guidance.BMC Dev Biol. 2008 May 28;8:59. doi: 10.1186/1471-213X-8-59. BMC Dev Biol. 2008. PMID: 18507827 Free PMC article.
-
Pax6 regulation of Math5 during mouse retinal neurogenesis.Genesis. 2009 Mar;47(3):175-87. doi: 10.1002/dvg.20479. Genesis. 2009. PMID: 19208436 Free PMC article.
-
PAX6 does not regulate Nfia and Nfib expression during neocortical development.Sci Rep. 2015 May 29;5:10668. doi: 10.1038/srep10668. Sci Rep. 2015. PMID: 26021864 Free PMC article.
-
Pax6 expressed in osteocytes inhibits canonical Wnt signaling.Mol Cells. 2013 Apr;35(4):305-12. doi: 10.1007/s10059-013-2310-0. Epub 2013 Mar 22. Mol Cells. 2013. PMID: 23529217 Free PMC article.
Cited by
-
Role of EGF receptor signaling on morphogenesis of eyelid and meibomian glands.Exp Eye Res. 2017 Oct;163:58-63. doi: 10.1016/j.exer.2017.04.006. Exp Eye Res. 2017. PMID: 28950938 Free PMC article. Review.
-
Genetic background-dependent role of Egr1 for eyelid development.Proc Natl Acad Sci U S A. 2017 Aug 22;114(34):E7131-E7139. doi: 10.1073/pnas.1705848114. Epub 2017 Aug 4. Proc Natl Acad Sci U S A. 2017. PMID: 28778995 Free PMC article.
References
-
- Callaerts P, Halder G, Gehring WJ (1997) PAX-6 in development and evolution. Annu Rev Neurosci 20: 483–532. - PubMed
-
- Collinson JM, Quinn JC, Hill RE, West JD (2003) The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev Biol 255: 303–312. - PubMed
-
- Kozmik Z (2008) The role of pax genes in eye evolution. Brain Res Bull 75: 335–339. - PubMed
-
- Grindley JC, Davidson DR, Hill RE (1995) The role of Pax-6 in eye and nasal development. Development 121: 1433–1442. - PubMed
-
- Ashery-Padan R, Gruss P (2001) Pax6 lights-up the way for eye development. Curr Opin Cell Biol 13: 706–714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

